Mycoplasma contamination in biopharmaceutical manufacturing
KEY POINTS
- Mycoplasma are ubiquitous, minuscule bacteria which can contaminate a bioprocess while being hardly detectable with standard methods such as cell culture or microscopic observation.
- Results of Mycoplasma contamination are typically poor process performance, low product yields, but can include more severe consequences that jeopardize patient safety.
- PCR testing is gaining traction as the preferred method for quick, specific and robust Mycoplasma testing, with a short turnaround time of hours, instead of days.
Contamination with adventitious agents poses a significant risk throughout the entire biopharmaceutical manufacturing process. Among these contaminants, Mycoplasma is particularly notorious and considered to be one of the most common and serious microbial contaminants associated with the production of biopharmaceuticals. Mycoplasma, a group which includes over 140 closely related species, are minuscule (0.15 – 0.3 µm) bacteria considered to be the smallest free-living organisms capable of self-replication. Mycoplasmas are devoid of cell wall, which makes them distinct from other bacterial species and not susceptible to antibiotics targeting cell wall synthesis (e.g. penicillins). Contamination rates in established cell cultures have been estimated to range from 15 to 35%, with notably higher incidence in mammalian and avian cell lines1, 2.
How can Mycoplasma be introduced to bioprocessing operation?
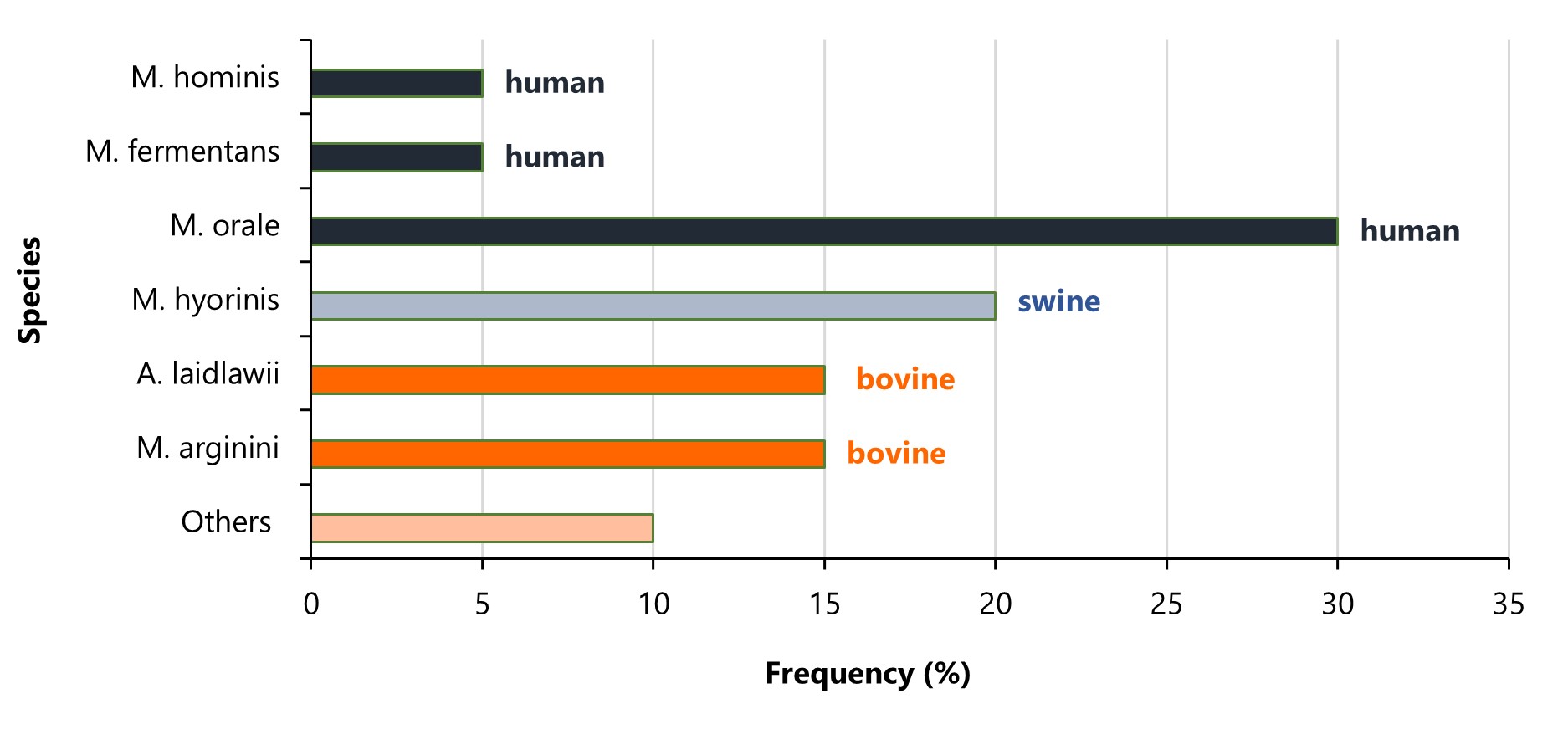
Mycoplasmas may contaminate raw materials, plastics, or cell lines used in a bioprocess3, 4. Owing to their minute size, these bacteria can easily pass through 0.1 µm sterilizing filters. Detection of Mycoplasma in cell culture poses a significant challenge since it does not impact turbidity and might go unnoticed under a microscope. The major sources of mycoplasma contamination are: personnel, incubators, liquid nitrogen, airborne particles, contaminated cell lines and insufficiently sterilized equipment5. Some Mycoplasma species, such as M.pneumoniae, are ubiquitous in humans and can be introduced into the process by the operator, making cell line development and upstream processing particularly vulnerable to contamination. More than 20 strains were isolated from infected cell lines with specific strains being responsible for the majority of infections. A total of 90-95% of contaminations are caused by 6 species: M. orale, M. fermentans and M. hominis from human, M. arginini and A. laidlawii from bovine and M. hyorinis from swine sources. Due to the fact that M. orale strain can be found primarily in the human oral cavity, this species is responsible for 20-40% of all cell line infections. Other strains that occur in humans and frequently infect the cell lines include M. fermentans and M. hominis. In addition to human-infecting species, mycoplasmas derived from cattle and swine also represent an important source of contamination and account for about 10-20% (the percentage of strains varies between studies) of infections6. The distribution of Mycoplasma contamination by species and their respective sources is shown in Figure 1.
Figure 1. Frequency and source of different Mycoplasma species contaminating cell cultures.
What are the consequences of Mycoplasma contamination?
Mycoplasma contamination poses significant risk to various aspects of biopharmaceutical production. It can alter DNA, RNA, protein synthesis, diminish amino acid and ATP levels, introduce chromosomal alterations, and modify host cell plasma membrane antigens, which can manifest in modified cell growth, reduced productivity, alteration of cellular morphology and/or changes in product characteristics3. Therefore, undiscovered Mycoplasma contamination essentially calls into question the significance and validity of the obtained results. Moreover, it can halt the recombinant protein production for months and require exhaustive and time-consuming decontamination process. When it comes to the impact on patient’s health, an undetected presence of mycoplasmas in the investigational or commercial medicinal product may cause sepsis and disseminated infection, which in some cases can lead to death7. Administration of contaminated batches during the clinical trial would undermine the quality of the manufacturing process and the implemented control measures, and in consequence impede the entire development program. For the outlined reasons, ICH, FDA and EMA guidelines require testing for the presence of Mycoplasma in cell/viral banks, cell harvest material and sometimes the product itself8, 9, 10, 11. These guidelines include:
- ICH Topic Q 5 D – Quality of Biotechnological Products: Derivation and Characterization of Cell Substrates Used for Production of Biotechnological/Biological Products
- Food and Drug Administration (FDA); “Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals”, 1993
- Food and Drug Administration (FDA); “Guidance for Industry Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications”, February 2010
- Food and Drug Administration (FDA); Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs), January 2020
- European Medicines Agency (EMA); Draft Guideline on quality, non-clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials (February 2019)
What is the best method for detecting Mycoplasma?
There are several methods available for detecting Mycoplasma contamination. They are described in European Pharmacopoeia Chapter 2.6.7 and United States Pharmacopoeia Chapter 63. The choice of the appropriate method depends on various factors, including the type of sample being tested and required sensitivity.
Traditionally, cell culture-based methods have been used with the tested sample inoculated into specialized growth media12. For strains that are difficult to grow, an indicator culture method may be utilized instead. Although this method is relatively simple, it can be time-consuming as Mycoplasma takes an average of 28 days to grow13.
In comparison to cell culture, qPCR-based methods are highly sensitive, rapid, and specific. These methods utilize specially designed primers targeting the highly conserved 16S rDNA gene, which are capable of detecting a wide range of Mycoplasma species14. Internal controls are incorporated to verify the success of reaction and lack of inhibition, and the results can be obtained within hours. While the detection limits for NAT-based methods must be at least 10 CFU/mL according to Ph. Eur. and USP, they often achieve lower levels, reaching as low as 0.1 CFU/mL for some strains15, 16. qPCR-based methods are gaining regulatory approval as a viable alternative to cell culture-based methods, and are especially important when the sample needs to be processed quickly, such as in the case of cell therapy products. Many NAT-based Mycoplasma detection assays have already been accepted by health authorities in the US, Europe and internationally, both for commercial and clinical lot release. There are several critical parameters an assay must fulfill in order to be compliant with the regulations set out by Ph. Eur. and USP. These are: specificity, sensitivity (limit of detection no higher than 10 CFU/mL) and robustness.
What can be done to avoid contamination?
To prevent Mycoplasma introduction, it is crucial to implement rigorous laboratory practices, including strict adherence to aseptic techniques, regular testing of cell lines and biological materials, proper decontamination of equipment and surfaces, and training personnel on good laboratory practices and hygiene. Regular screening of cell lines and vigilant monitoring can help detect and promptly mitigate the risk of contaminating the final drug product. Testing of biopharmaceutical products and half-products (such as harvest, cell banks and sometimes the final DP) for Mycoplasma contamination is required by the EU, US and UK regulatory agencies. Nucleic acid-based assay alone or in combination with cell culture are acknowledged as appropriate detection methods. As stated in one of the FDA guidances on viral vaccines17:


PCR-based assays may be used to detect mycoplasma, provided that such an assay can be shown to be comparable to the agar and broth media procedure and the indicator cell culture procedure. Such assays may combine culture and PCR. In some cases, culture-based procedures cannot be used due to an inability to completely neutralize vaccine viruses, thus necessitating the use of PCR-based assays to test for mycoplasma in these products.


In summary, Mycoplasma contamination poses a serious concern for biopharmaceutical manufacturers, as it represents both safety and economic risks. Routine testing for Mycoplasma can help minimize these dangers, with qPCR being the fastest detection method showing outstanding reproducibility, sensitivity and ability to identify non-culturable strains. Proper implementation of the routine Mycoplasma testing under the GMP requirements requires prior method validation. Mabion offers a validated qPCR method together with specific sample suitability testing as well as routine testing for Mycoplasma contamination.
Click here to learn more about our Mycoplasma testing services.
Adam Tuszyner
Nina Durys
Literature
- Shayn E Armstrong 1, Jill A Mariano, Daniel J Lundin. The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals 2010 Mar;38(2):211-3. doi: 10.1016/j.biologicals.2010.03.002 ↩︎
- Ceccherini-Nelli, Luca, and Barbara Matteoli, eds. Biomedical Tissue Culture. BoD–Books on Demand, 2012. ↩︎
- Rottem, S., & Naot, Y. Subversion and exploitation of host cells by mycoplasmas. Trends in Microbiology 1998 6(11), 436–440 ↩︎
- Angart P, Kohnhorst C, Chiang M, Arden NS, Adventitious agents are a safety risk to biomanufacturing pipelines. USP 33/NF 28 Mycoplasma Tests. (2018, October 25). Considerations for risk and control of Mycoplasma in bioprocessing. Current Opinion in Chemical Engineering ↩︎
- Prevention and Detection of Mycoplasma Contamination in Cell Culture. Laleh Nikfarjam, Ph.D.* and Parvaneh Farzaneh, Ph.D . Cell J. 2012 Winter; 13(4): 203–212 ↩︎
- Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Hans G. Drexler∗ & Cord C. Uphoff DSMZ-German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschweig, Germany ↩︎
- Tawfik P. & Patrick Arndt. Lethal hyperammonemia in a CAR-T cell recipient due to Ureaplasma pneumonia: a case report of a unique severe complication. BMJ Case Rep. 2021;14(7):e242513. Published 2021 Jul 8. doi:10.1136/bcr-2021-242513 ↩︎
- European Medicines Agency (EMA); Draft Guideline on quality, non-clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials (released for public consultation in February 2019) ↩︎
- Food and Drug Administration (FDA); Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs), January 2020 ↩︎
- Food and Drug Administration (FDA); “Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals”, 1993 ↩︎
- ICH Topic Q 5 D – Quality of Biotechnological Products: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products ↩︎
- Janetzko K., Rink G., Hecker A., Bieback K., Klüter H., Bugert P. A single-tube real-time PCR assay for Mycoplasma detection as a routine quality control of cell therapeutics. Transfusion Medicine and Hemotherapy 2014 Feb; 41(1): 83-99. doi: 10.1159/000357096. ↩︎
- Young L, Sung J, Stacey G, Masters JR (2010) Detection of Mycoplasma in cell cultures. Nature Protocols, doi:10.1038/nprot.2010.43 ↩︎
- Jean A, Tardy F, Allatif O, Grosjean I, Blanquier B, Gerlier D (2017) Assessing mycoplasma contamination of cell cultures by qPCR using a set of universal primer pairs targeting a 1.5 kb fragment of 16S rRNA genes. PLoS ONE 12(2): e0172358. doi:10.1371/journal.pone.0172358 ↩︎
- European Pharmacopoeia, Chapter 2.6.7 “Mycoplasmas” ↩︎
- US Pharmacopoeia <63> “Mycoplasma Tests: A New Regulation for Mycoplasma Testing” ↩︎
- Food and Drug Administration (FDA); “Guidance for Industry Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications”, February 2010 ↩︎