Science News
Discover the news and inspiring materials from the world of biotechnology.

Mabion Launches €500,000 Services Contest to Support Next-Gen Oncology Breakthroughs
At Mabion we understand how to advance promising biosimilars and novel biotherapeutics to the clinic and from there to patients. Unfortunately, many promising biopharmaceutical innovations spanning novel recombinant protein therapeutics to biosimilars are being held back in the current, limiting funding climate, depriving patients from access to advanced therapies. Yet the urgency to close gaps in unmet patient care has never been greater.That is why we are launching the “Unlocking the Future of Therapeutics” competition a unique opportunity to support and accelerate biologics development and scale up for an oncology-targeted therapeutic.We are offering €500,000 credit in development services to support an exceptional therapeutic project. This can be a novel therapeutic, but might also be a biosimilar drug which expands patients access to lifesaving care.We have partnered with respected academic experts focused on cancer research to select the most promising candidate.
Learn more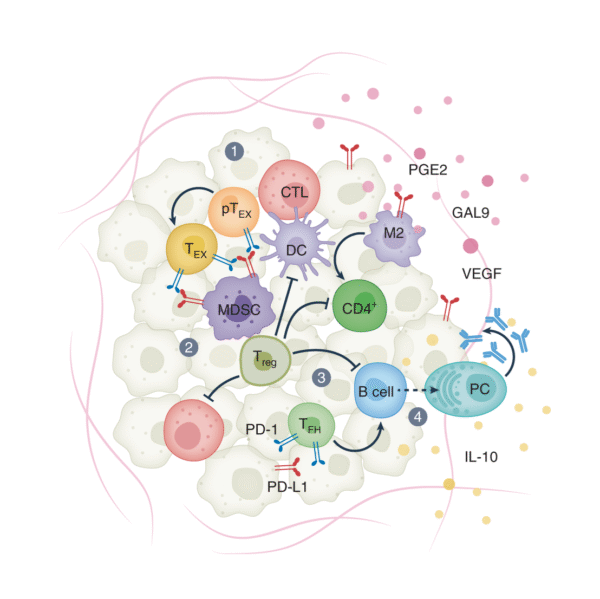
Melanoma Immunotherapy Enhance by Immunity Activation
By reintroducing the Interleukin-4 into IFN-I–dominant, inflammation-deficient tumors, scientists successfully reinvigorated T cell–driven tumor rejection. Whether introduced directly or delivered via engineered T cells in ACT, IL-4 helped reshape the immune landscape, boosting effector T cells and improving survival in mouse models.
Learn more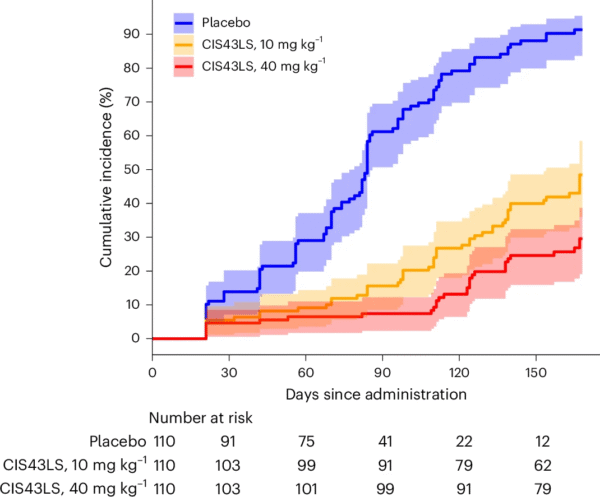
Single-Dose Monoclonal Antibody Therapy Offers Durable Protection Against Malaria
Monoclonal antibody CIS43LS reduced the cumulative incidence of infection by 87.4% at 40 mg/kg during the 6-month qRT-PCR monitoring period. This Phase 2 trial evaluated its efficacy in 330 adults in Mali during a 6-month high-transmission season.CIS43LS is a human IgG1 monoclonal antibody targeting the circumsporozoite protein of Plasmodium falciparum, designed to confer long-acting protection with a single intravenous dose.
Learn more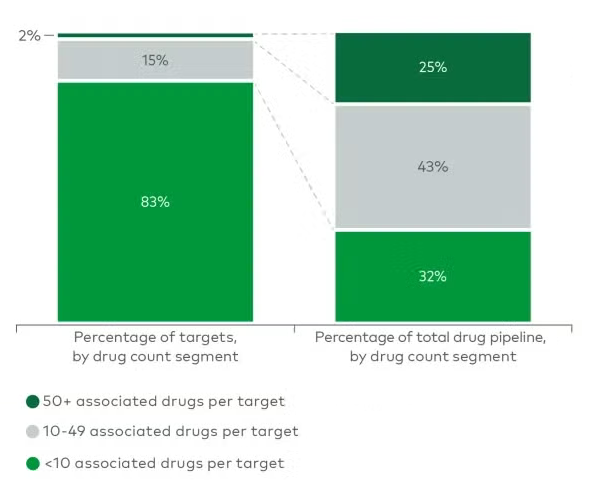
Biopharma Drug Development Gaps Strategy
Why biopharma drug development is stalling around just 38 targets? Novel targets decline as biopharma companies favors the familiar ones. Expert report reveals risk-averse shift in biopharma pipeline. Known targets offer efficiency, predictability, and infrastructure. Target crowding threatens innovation in Biopharma Drug Development.
Learn more
Winning Trust Through Mabion Biologics CDMO Excellence – exclusive interview with Marty Henehan
What does it take to stand out as a global biologics CDMO? In this interview, Marty Henehan shares insights from DCAT Week, discusses Mabion’s award-winning approach to customer partnerships, and reveals how the company is shaping the future of biologics manufacturing.
Learn more
Mabion is a Champion of the 2025 International CDMO Leadership Award in Biologics
Mabion Wins as the Champion of the 2025 International CDMO Leadership Award in Biologics! This achievement is a testament to our commitment to innovation, reliability, and excellence in biologics development and manufacturing. We extend our heartfelt thanks to our clients for their trust and collaboration – your success is our success!
Learn more
Mabion’s Fill & Finish services in Contract Pharma Magazine
The Fill & Finish stage of pharma manufacturing may not make headlines, but it is the final crucial step – the point where all the work behind a new drug either comes together or falls apart due to contamination, inefficiencies, or regulatory setbacks. Right now, the demand for high-quality, adaptable fill-finish services is soaring. Mabion Biologics CDMO offer the expertise, flexibility, and advanced technology that pharmaceutical companies need to navigate today’s challenges. Explore our Production Division Manager’s expertise on the role of compliance in today’s filling and finishing landscape in Contract Pharma.
Learn more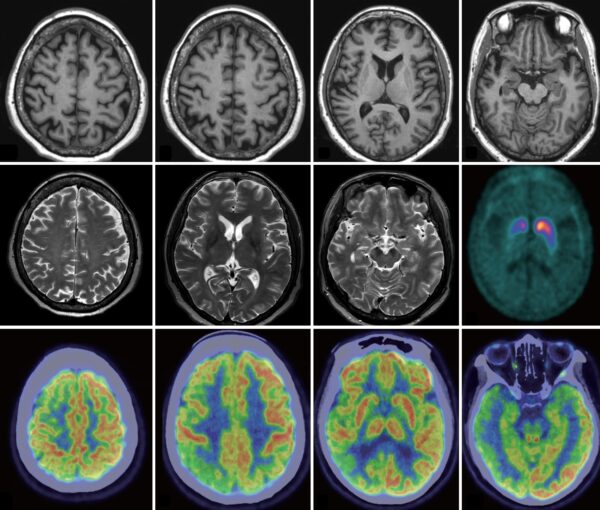
Dyskinesias in Parkinson’s Disease: Promising New Treatment Approach
CPL’36 Phase II clinical trial results reinforce the potential of PDE10A inhibition as a new therapeutic strategy for managing dyskinesias and improving the quality of life for individuals with Parkinson’s disease.
Learn more