Science News
Discover the news and inspiring materials from the world of biotechnology.

Mabion is a Finalist in the CDMO Leadership Awards 2025 in the Biologics category
Winning the 2025 CDMO Leadership Awards strengthens Mabion’s reputation as a high quality CDMO, making us more attractive to potential clients. The recognition will boost our industry presence, opening doors to new business opportunities and partnerships.
Learn more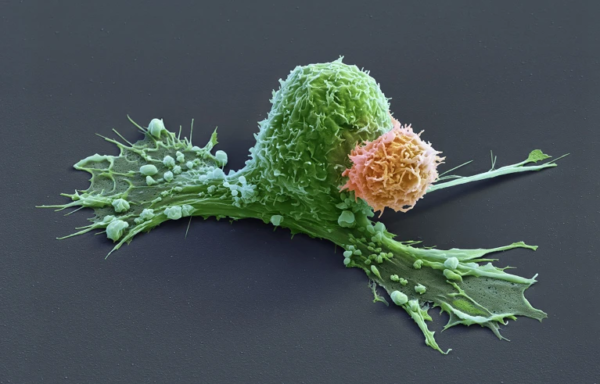
How long might CAR-T cell therapy work?
In a remarkable medical milestone, a woman has remained cancer-free for an unprecedented 19 years following a pioneering CAR-T cell therapy administered during her childhood. This groundbreaking case offers renewed hope for the long-term efficacy of CAR-T cell treatments in combating rare pediatric cancers.
Learn more
Mabion Wins Best of Industry Insights Award for Biologics Outsourcing Article in Outsourced Pharma Best Of 2024
Mabion SMEs’s article has been awarded Best of Industry Insights in the Biologics Outsourcing category of the Outsourced Pharma Best Of 2024 awards.
Learn more
FDA approves first medication for obstructive sleep apnea: Eli Lilly’s Zepbound GLP-1 agonist
The FDA has approved Eli Lilly’s Zepbound (tirzepatide) as the first-ever medication for treating moderate to severe obstructive sleep apnea (OSA) in adults with obesity, providing a pharmacological alternative to CPAP machines and surgery.
Learn more
Biocon’s Yesintek receives EMA and FDA approval as the next ustekinumab biosimilar
Yesintek, a biosimilar to ustekinumab developed by Biocon, has received approval from both the EMA and FDA, joining other ustekinumab biosimilars like Amgen’s Wezlana and Alvotech’s Selarsdi.
Learn more
Lecanemab approved by EMA in early Alzheimer’s after re-evaluation
The European Medicines Agency (EMA) reversed its initial rejection and approved lecanemab (Leqembi) for early-stage Alzheimer’s, limiting its use to patients with one or no copies of the ApoE4 gene. The restriction of indication is intended to maximize the benefit-risk ratio of the new drug.
Learn more
Transplantation of stem cells islets cures patient from type 1 diabetes
Chinese scientists have reversed Type 1 diabetes in a patient by transplanting chemically-induced pluripotent stem-cell-derived islets (CiPSC islets), marking a significant medical breakthrough. The procedure reprogrammed the patient’s own fat cells into insulin-producing islets, eliminating the need for insulin therapy and reducing the risk of immune rejection frequently seen with donor-based transplants. Stable glycemic control was maintained at least for one year, with no registered complications.
Learn more
Nobel prize in medicine awarded to microRNA discoverers
This year’s Nobel prize in physiology or medicine has been awarded to Victor Ambros and Gary Ruvkun who discovered microRNAs (miRNAs) and elucidated their role in regulating gene expression. MicroRNAs are a class of small RNA molecules that reduce the expression of specific genes by binding to and disrupting their corresponding mRNAs. This regulation of gene expression pattern by miRNAs plays a key role in driving cell differentiation and determining cell fate. The discoveries of this year’s Nobel laureates are likely to pave the way for novel cancer therapies.
Learn more