IL-4/IFN-I Activation to Enhance Melanoma Immunotherapy
- Immunotherapy Interleukin-4 (IL-4) supplementation renews T cell immune functions in resistant IFN-I–dominant melanoma.
- The future of melanoma treatment may depend on leveraging IFN-I to activate immune responses, interleukins to restore balance and resilience, and checkpoint inhibitors to lift immune suppression.
- In adoptive T cell therapy (ACT) models, tumor-specific T cells engineered to express IL-4 showed significantly improved tumor control and survival, demonstrating IL-4’s ability to function as an immune enhancer when used to complement excessive interferon activity.
Western Australia’s capital, Perth, has for years been regarded as a leading continental center for cancer research and innovative therapies, thanks to the work of experts at the Harry Perkins Institute of Medical Research. A wealth of scientific experience combined with ambitious projects and access to cutting-edge technology allows us to push the boundaries of cognition and discover new biological connections in our bodies. It was in a consortium from here in the world, including the University of Western Australia, Harry Perkins Institute of Medical Research and The Kids Research Institute Australia (with support from Sir Peter MacCallum Department of Oncology, The University of Melbourne for plasmid preparation), that a potentially groundbreaking immune system stimulation for cancer treatment was discovered.1
Effects of Interferons and Cytokines on the Immune System
Interferons (IFNs) have played a significant role in the history of melanoma therapy, initially used as adjuvants in high-risk patients following surgical resection. Type I IFNs, particularly IFN-α, have demonstrated clinical benefit in reducing relapse in operable melanoma and enhancing immune surveillance through their antiviral and immunomodulatory properties. Despite these advantages, the use of high-dose systemic IFN therapy has been limited due to its toxicity profile and variable efficacy. In melanoma, IFN-γ can exert both anticancer effects associated with cell-cycle arrest and cell death induction and protumorigenic activity related to immune evasion leading to melanoma progression.2
Interleukin-4 (IL-4) is a cytokine traditionally associated with type 2 immune responses, particularly in allergy and helminth infection, but recent research has highlighted its nuanced role in cancer immunology. Within the tumor microenvironment (TME), IL-4 exerts significant influence over immune cell differentiation and function, increasing effector-like T cells and rebalancing macrophage and fibroblast populations. This suggests, in the context of interferon-rich, inflammation-skewed tumors (such as certain melanomas) IL-4 appears to serve a balancing function. When IFN-I signaling is dominant and type 2 cytokines are depleted, CD8+ T cells often become dysfunctional or exhausted. Introducing IL-4 in these cases has been shown to rescue effector T cell activity, modulate macrophage profiles, and restore immune surveillance capabilities, thereby enhancing antitumor immunity.
IL-4/IFN-I Combination Melanoma Immunotherapy
Recent preclinical models of melanoma have deepened our understanding of how type I IFNs operate within the tumor immune context. Notably, certain IFN-I subtypes, such as IFNα4, can trigger robust interferon-stimulated gene activation without inducing durable tumor rejection. In these scenarios, the tumor microenvironment is skewed toward excessive interferon signaling and depleted of type 2 cytokines such as IL-2, IL-4, and IL-13.3 This imbalance leads to impaired CD8+ T cell effector function, defective memory T cell formation, and reduced activity of immune-regulating cell types like M2 macrophages and ILC2s.
Clinically, the IL-4/IFN-I combination has the potential to serve as a foundation for more personalized melanoma immunotherapies. By stratifying patients based on their tumor’s immune transcriptomic profile clinicians could identify those most likely to benefit from this synergistic approach. Furthermore, combining IL-4/IFN-I therapy with PD-1/PD-L1 inhibitors may amplify therapeutic responses, enhancing T cell reinvigoration while suppressing tumor-intrinsic immune evasion.4


Understanding how immune signals interact inside tumors opens new doors to help patients who currently have few options.


Correcting Cytokine Imbalance Through IL4 Improves Melanoma Immunotherapy
Analysis of melanoma patient datasets revealed that IFN-I↑ T2↓ signatures correlated with poor clinical outcomes. This phenotype, identifiable through transcriptomic profiling, could serve as a biomarker for patient stratification and personalized immunotherapy design. The combination of IL-4 supplementation with existing ICB strategies offers a promising route for hard-to-treat cases. When IL-4 is introduced into this IFN-I↑ T2↓ environment – either through ectopic expression or adoptive cell transfer – tumor control improves significantly. This demonstrates that optimal immunotherapy may depend not only on activating the immune system but also on carefully rebalancing it.
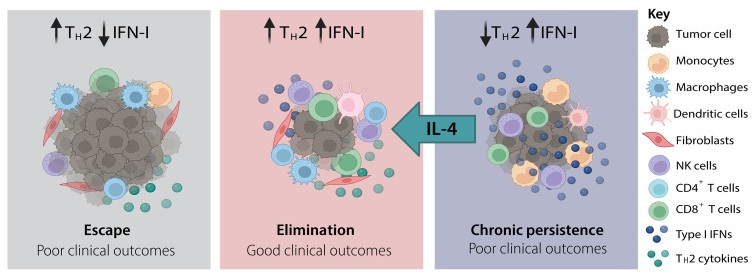
Figure 1. Diagram showing three tumor phenotypes driven by IFN-I and type II inflammation in tumor microenvironment.
IL-4 acts through its receptor complexes (type I and type II IL-4 receptors), which activate the STAT6 signaling pathway and regulate genes involved in immune cell survival, proliferation, and function. In cancer models where type I interferon activity suppresses type 2 inflammation, IL-4 supplementation has helped reprogram the TME into a more permissive landscape for T cell activation and tumor clearance. It enhances the development of effector phenotypes in CD8+ T cells and may contribute to the recruitment or maintenance of cell types like ILC2s and M2-like macrophages, which play more complex, context-dependent roles in tumor biology.
The IFN-I↑ T2↓ molecular phenotype could serve as a biomarker to stratify patients unlikely to benefit from conventional PD-1 inhibitors alone and better suited for combination approaches.5 Moreover, it raises caution about the use of IFN pathway inhibitors – such as JAK blockers or anti-IFNAR antibodies – in patients with melanoma. Especially in those undergoing immune checkpoint therapy, as these agents could inadvertently suppress beneficial IFN-driven immune priming.
Prepared by:

Jakub Knurek
Marketing Specialist
Sources and further reading
- Newnes HV, Armitage JD, Buzzai AC, de Jong E, Audsley KM, Barnes SA, Srinivasan S, Serralha M, Fear VS, Guo BB, Jones ME, Forrest ARR, Foley B, Darcy PK, Beavis PA, Bosco A, Waithman J. Interleukin-4 modulates type I interferon to augment antitumor immunity. Sci Adv. 2025; 11(20): eadt3618.
- Wawrzyniak P, Hartman ML. Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors. Mol. Cancer. 2025; 24: 89.
- Im SJ, Lee K, Ha SJ. Harnessing IL-2 for immunotherapy against cancer and chronic infection: a historical perspective and emerging trends. Exp Mol Med. 2024; 56(9): 1900-1908.
- Razaghi A, Durand-Dubief M, Brusselaers N, Björnstedt M. Combining PD-1/PD-L1 blockade with type I interferon in cancer therapy. Front Immunol. 2023; 14: 1249330.
- Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022; 23(5): 660-670.