Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing
- Time to market is a strategic imperative in biologics. Only 1 in 10,000 drug candidates reaches the market, making every day in development count. Being first to launch offers unmatched business advantages in pricing, prescriber preference, and long-term market share.
- CDMO offers biopharmaceutical companies immediate access to infrastructure and expertise. This accelerates progress through the development stages and eliminates additional costs. It allows smaller sponsors to operate like mature pharmaceutical organizations without excessive internal resources.
- Outsourcing to a CDMO improves control over key time-to-market metrics. From cell line development time to regulatory documentation readiness, experienced service providers compress timelines across the board. With proven systems, multidisciplinary teams, and regulatory foresight, CDMOs enable biotech companies to stay ahead in crowded, fast-moving therapeutic markets.
Time to market is a defining benchmark in the development of biologic drugs, where scientific rigor must align with operational efficiency to translate innovation into therapeutic availability. Whether developing monoclonal antibodies, fusion proteins, bispecifics, ADCs, or protein-based vaccine antigens, biotech companies face a technically demanding path that involves complex cell line engineering, multi-step purification protocols, method qualification, and global regulatory navigation.
Each modality presents unique challenges: monoclonal antibodies require tight control of glycosylation profiles and aggregation levels, bispecifics demand innovative expression systems and analytical strategies, and vaccine antigens must meet rigorous potency, safety, and stability standards—often within compressed timelines dictated by public health needs or competitive pressures.
In this landscape, a CDMO company plays a critical role by providing streamlined development pathways based on platform processes, integrated analytics, and manufacturing systems already aligned with regulatory expectations.
What Is Time to Market and Why Does It Matter for Biologics Launches?
Time to market (TTM) refers to the total time it takes to advance a biologic drug candidate from discovery through clinical development, regulatory approval, and commercial launch. For biotech companies developing monoclonal antibodies, protein vaccine antigens, or other biologics, accelerating time to market is financial metric and strategic imperative.
In the biologics market, being first to launch a therapeutic against a specific target or disease mechanism often delivers outsized rewards. First-mover biologics typically gain:
- Market exclusivity and physician preference
- Early reimbursement traction
- Brand recognition that persists even after biosimilars emerge
Additionally, in areas like oncology, immunology, and rare diseases, unmet medical needs are so high that the first available therapy can dominate prescribing patterns and secure a loyal prescriber base long before competitors arrive.1
For small and mid-sized biotech companies, shortening time to market also improves capital efficiency. Early revenues support further R&D and reduce dilution from external financing. However, there is a very high risk here. Only one in 10,000 potential drug candidates makes it to the market as a new treatment.2
Working with an experienced CDMO company can help mitigate that risk by accelerating timelines and ensuring regulatory alignment from early development through to launch.
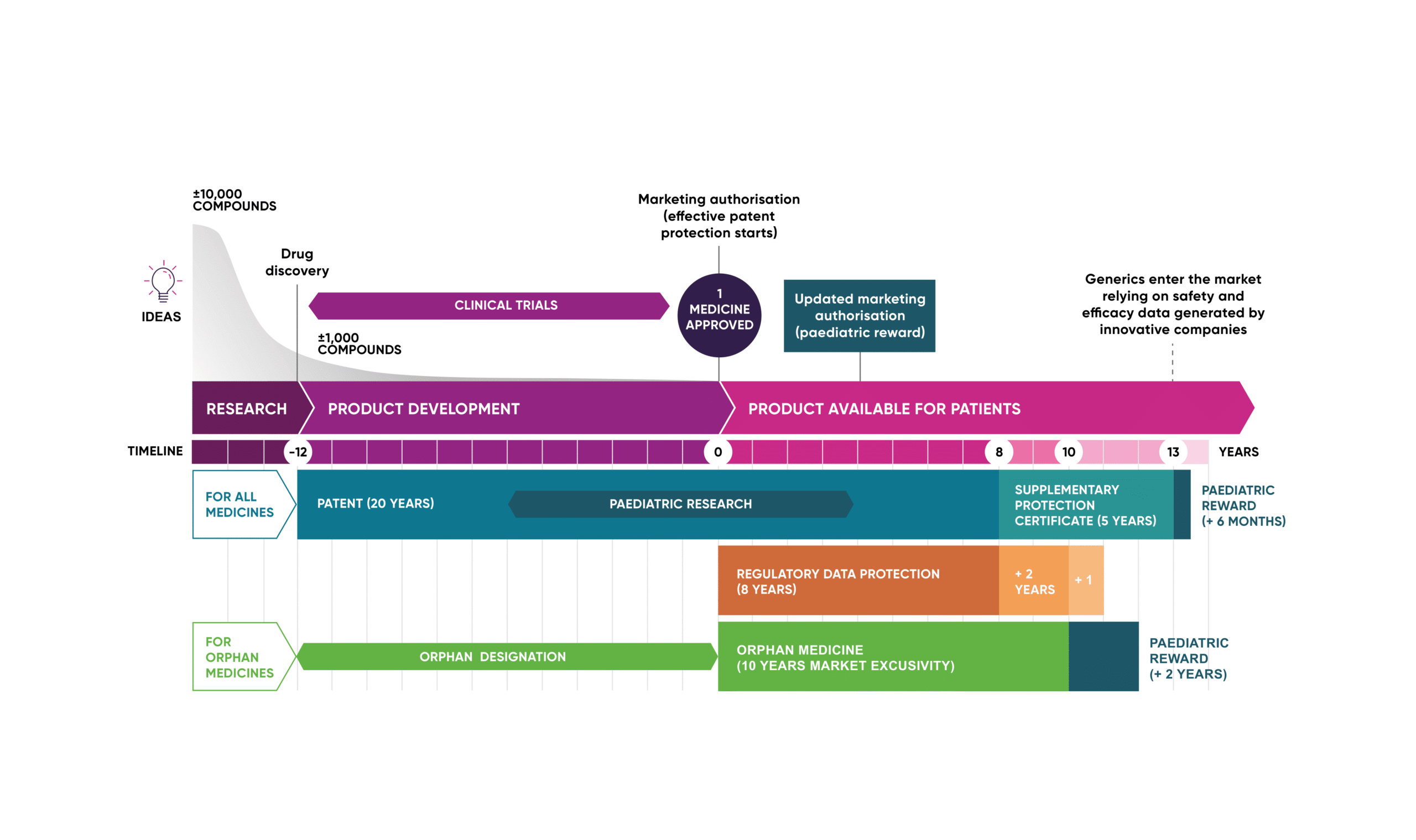
It takes 8-10 years to bring a drug to market and the best estimates suggest that only around 1 in 10,000 candidates successful pass the finishing line. However, the game is worth the candle, as exemplified by success stories.
| Therapy | Approval date | Indication | Target | Peak Annual Revenue |
| MabThera (rituximab) | 1997 | Non-Hodgkin’s lymphoma | CD20 | $8.6 billion (2016)4 |
| Herceptin (trastuzumab) | 1998 | HER2-positive metastatic breast cancer | HER2 | $7 billion (2019)4 |
| Humira (adalimumab) | 2002 | Rheumatoid arthritis | TNF-α | $21.2 billion (2022)5 |
| Avastin (bevacizumab) | 2004 | Metastatic colorectal cancer | VEGF-A | $7.1 billion (2018)4 |
| Keytruda (pembrolizumab) | 2014 | Advanced melanoma | PD-1 | $29.5 billion (2024)6 |
Table 1. First-to-Market Biologics and Their Commercial Success.
How CDMOs Can Accelerate Your Biologic Development Process?
For biotech companies advancing biologic drug candidates, time to market is a defining metric of success. The journey from early development to commercial approval is complex, capital-intensive, and tightly regulated. That factors can overwhelm small or mid-sized organizations without extensive internal infrastructure. In this context, partnering with a CDMO allows biotech firms to focus their internal resources on innovation and clinical strategy, while leveraging external expertise to execute the technical and regulatory pathways efficiently.
Service providers bring purpose-built facilities, specialized talent, and established quality systems that are difficult and time-consuming to build in-house. Their ability to manage end-to-end development and manufacturing within a unified framework eliminates fragmentation and reduces the risk of delays caused by vendor transitions or process misalignment. As a result, biotech companies can maintain momentum across development phases and move more confidently through key milestones. In today’s biologics landscape, speed is often the basis for market leadership. CDMO partnerships enable emerging biotech firms to act with the scale, rigor, and agility of much larger players.
5 Key Tactics CDMOs Use to Shorten Biologic Time to Market
Tactics used by CDMOs to reduce time-to-market refer to coordinated activities, systems and technical frameworks that allow them to move biological products through development stages faster than individual sponsors. The term “tactics” is essentially a cumulative advantage of operational discipline, access to technology, and regulatory foresight, used for the specific purpose of accelerating schedules. CDMOs leverage their advanced platforms, proven manufacturing templates, integrated analytics and experienced regulatory teams to transform scientific workflows into streamlined manufacturing pathways. They are ahead of biotech companies with ready-to-implement technologies, proven processes and multidisciplinary teams that operate in fully GMP-compliant environments.
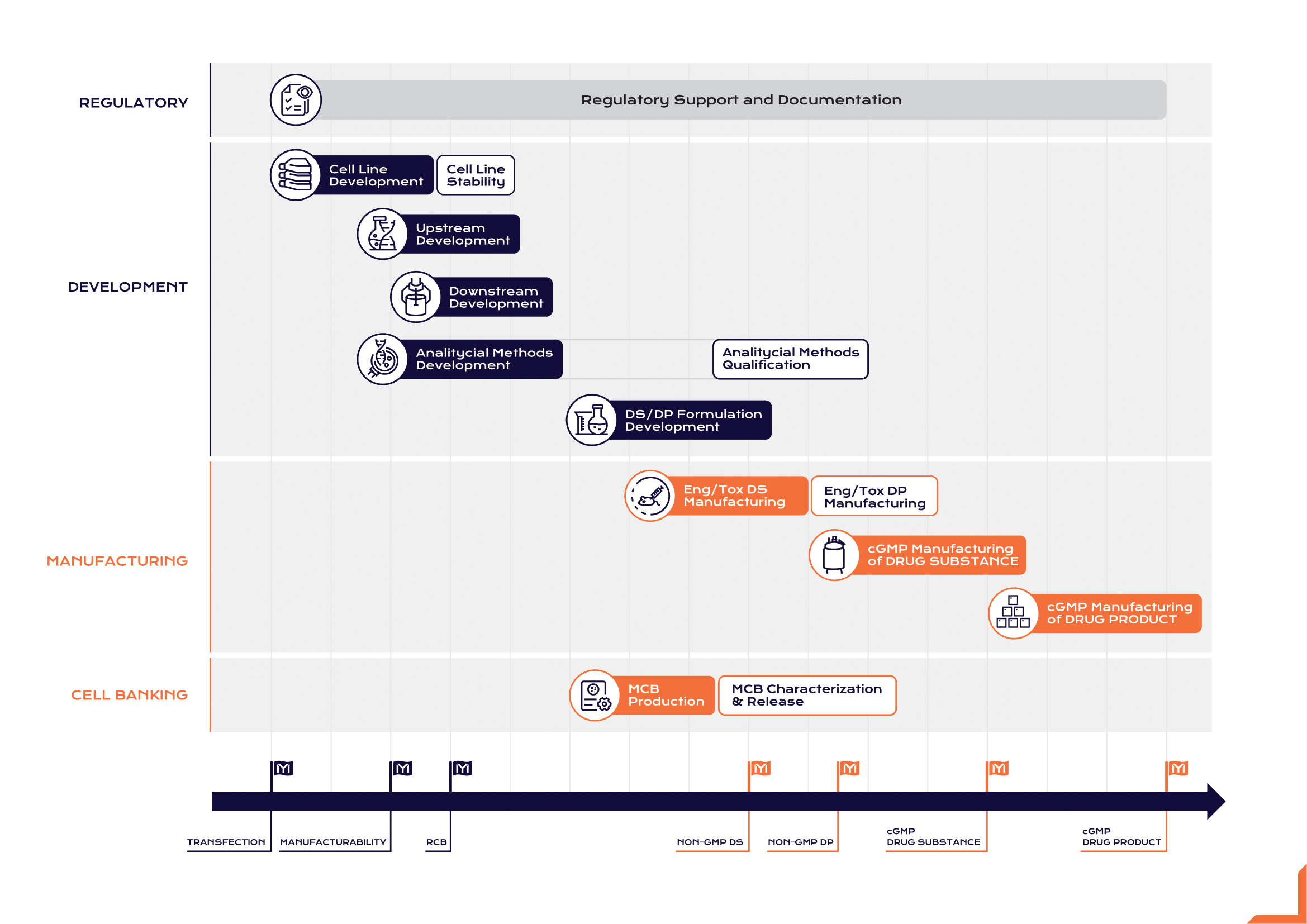
Integrated End-to-End Services
A vertically integrated structure enables clients to accelerate biologic drug development by centralizing process development, analytical characterization, GMP manufacturing, and fill-finish within a unified quality and documentation system. This eliminates typical delays associated with outsourcing to multiple providers and facilitates a seamless technology transfer process across project phases. From cell line development through clinical and commercial supply, we ensure scientific consistency and regulatory traceability.
Our company’s ability to integrate development and manufacturing is underpinned by close collaboration with Sartorius, Repligen, and Cytiva, leveraging advanced upstream platforms, single-use bioreactors, and downstream chromatography systems. These collaborations give Mabion access to modular, scalable, and regulatory-ready systems that reduce batch-to-batch variability and allow rapid scale-up without revalidation of critical parameters.
Platform Processes
Mabion applies platform process strategies across monoclonal antibodies and recombinant protein production to accelerate early-stage development. By utilizing standardized upstream and downstream configurations, Mabion reduces development variability, shortens process design cycles, and ensures alignment with EMA and FDA expectations for both clinical and commercial supply.
In the upstream platform, Mabion uses CHO-based expression systems supported by scalable single-use bioreactors provided through its partnership with Cytiva and Kühner.9,11 The partnership with Bruker Cellular Analysis guarantees 100% confidence in the monoclonality of the derived cell lines.8 The platform covers a defined sequence of steps:
- Cell line development in Beacon Select™ Optofluidic System
- Cell line adaptation and banking
- Fed-batch culture in lab-scale bioreactors
- Nutrient feed strategy optimization
- Real-time control of critical parameters
Mabion employs high-throughput clone screening and scalable culture media, allowing rapid selection of high-producing clones with consistent glycosylation and protein integrity.12 Inline sensors and PAT tools allow early optimization of cell metabolism and productivity, enabling seamless tech transfer from small scale to GMP production. Having two bioreactor technologies is also a huge advantage: Stirred Tank Bioreactors (Cytiva)9 and Orbital Shaking Bioreactors (Kühner).11
The downstream platform integrates Mabion’s chromatography-based purification and polishing processes, designed for high yield, purity, and scalability. It includes:
- Cell culture harvest using depth filtration or centrifugation techniques
- Protein A affinity chromatography for monoclonal antibodies interception from medium
- Viral clearance and inactivation using in depth and tangential-flow filters to enhance the safety of biotherapeutic products
- Ion exchange chromatography (IEX) – both cation-exchange chromatography (CEX) and anion-exchange chromatography (AEX) available, and depending on project needs
- Hydrophobic interaction chromatography (HIC) or weak-cation exchange chromatography (WCX)
- Mixed-mode chromatography (MM) combines hydrophobic charge and ion exchange separation mechanisms within a single chromatographic column to enhance selectivity and purification efficiency
- Size exclusion chromatography (SEC) for final polishing
- Ultrafiltration (UF) or diafiltration (DF) to increase and optimize drug concentration
- Drug Formulation
Mabion optimizes drug recovery step, resin lifecycles, and buffer formulation using Design of Experiments (DoE) and in-process analytical testing. Critical quality attributes such as purity, charge variants, and aggregation levels are monitored at each step using qualified analytical methods. This results in a highly reproducible platform that supports regulatory-compliant batch documentation and reduced batch-to-batch variability.
Furthermore, platform-based manufacturing aligns with the reuse of scalable process units and automation-ready workflows enhances predictability and reduces variability, which is particularly valuable for clients moving rapidly from preclinical to clinical phases.13
Advanced Analytical Portfolio
Analytical capabilities represent one of the CDMO industry core competitive advantages in accelerating biologic development. Mabion has established a diverse portfolio of 40+ qualified methods for physicochemical characterization, protein structure analysis, functional bioassays, and impurity profiling. This scientific breadth supports comprehensive comparability and stability studies for biosimilars required for regulatory filings.
Our capabilities include state-of-the-art techniques such as high-resolution mass spectrometry (LC-MS) for peptide mapping and post-translational modification analysis, circular dichroism for protein secondary and tertiary structure conformation, UHPLC-based glycan profiling, CE-SDS and SEC for aggregation and purity studies, and evaluation of product- and process-related impurities using several orthogonal analytical methods. Mabion also operates a dedicated suite of cell-based bioassays including ADCC, CDC, and Fc receptor binding using platforms like SPR, ELISA, and flow cytometry to confirm biological activity and receptor specificity.14 Our facilities are equipped for both early-phase and commercial-phase GMP testing, including release studies, raw material testing, and biosafety assessments (e.g., endotoxin, bioburden, mycoplasma).15 We use market standards to conduct our analysis, which ensures high quality and repeatable results. By working with qualified suppliers such as Eppendorf, Agilent Technologies, Promega, Argenta, Corning, Bio-Rad and MilliporeSigma, we are assured that the materials and equipment we use to conduct projects for our clients are the best available.
Mabion’s integrated analytics are also designed for rapid data reporting and batch release. The in-house bioassay lab enables precise evaluation of biological activity (e.g., via cell-based potency assays), and the company’s adherence to ICH Q6B and Q2(R1) guidelines ensures global regulatory alignment.16,17 With deep understanding of EMA, FDA, Ph. Eur., and ICH guidelines, Mabion ensures its data meets global standards. This enables clients to submit robust data packages earlier and with higher confidence, directly impacting regulatory review timelines.
Single-Use Technologies
These technologies enable flexible, scalable, and contamination-resistant production environments. The use of single-use stirred tank bioreactors for cell culture and versatile chromatography systems for purification allows for rapid batch execution without complex cross-validation steps. Disposable bioreactors and pre-sterilized fluid paths reduce cleaning validation burdens, setup time, and changeover requirements — critical elements for shortening manufacturing lead times.
This technology was vital during the Novavax vaccine project, where rapid changeovers, high sterility assurance, and flexibility in production scale were essential.18 Mabion’s ability to integrate single-use systems into its GMP suites not only improved speed but also mitigated microbial contamination risks, providing assurance to regulatory authorities and enabling rapid product release.
Early Phase Manufacturing Readiness
GMP-certified manufacturing infrastructure is optimized for early-phase production, enabling rapid transition from process development to clinical trial material supply. Dedicated suites and a modular approach allow the company to initiate manufacturing campaigns with minimal delay. This readiness is especially valuable to clients preparing for the Biologics License Application (BLA), Investigational New Drug (IND) or Investigational Medicinal Product Dossier (IMPD) filings with tight timelines.19
The company’s integrated project teams and readiness assessments ensure all critical functions – QA, QC, QP, supply chain, engineering, and production – are aligned before GMP batches are initiated. Equipment qualified under platform protocols can be deployed with minimal delay, allowing early production campaigns to begin soon after process lock-in.
This capability proved essential during the vaccine production project, where speed and compliance were non-negotiable. Mabion’s readiness to manufacture within a compressed schedule demonstrated its capability to support time-critical public health goals. For clients entering the clinic or scaling to commercial production, Mabion offers scientific reliability and operational speed under one roof.
Leveraging Technology and Innovation to Fast-Track Biologic Production
Fast-tracking biologics production relies on process designs that emphasize standardization, optimization, and scientific predictability across the entire development lifecycle. By applying platform-based approaches programs can avoid redundant process development and move rapidly from early-stage expression to clinical-grade material. Optimized feed strategies, consistent media formulations, and standardized chromatography sequences reduce variability and minimize the number of experimental iterations needed to achieve target product quality.
Throughout the manufacturing process, technologies that enable short setup times, minimal hold steps, and high step recovery significantly reduce production cycle durations. Buffer preparation, filtration, and formulation are streamlined using pre-qualified components and harmonized protocols that support multiple molecules with minimal customization. These strategies not only accelerate lot execution and reduce downtime between batches, but also simplify validation and comparability efforts making it possible to meet compressed clinical timelines and transition more efficiently into commercial supply.
In 2021, Mabion began by implementing a platform-based process framework using a pre-qualified CHO expression system and purification strategy. This eliminated the need to design a process from scratch at an early stage and allowed the team to focus on molecule-specific customizations. Using a single-use bioreactor platform, production setup time was reduced by more than 30% compared to traditional stainless steel systems. Overall, by adding minor downstream improvements, the estimated time to release of the first GMP batch was reduced by 40%. This makes it possible to move to clinical manufacturing several months ahead of the sponsor’s internal projections. Process reproducibility has improved significantly, with a threefold decrease in deviations, thanks to tighter control over critical quality attributes and in-process analytics.
The successful execution of this project validated Mabion’s innovative model as a scalable solution to accelerate biologic drug development programs. The integration of platform technology, modular operations and scientifically grounded process design enhances CDMO’s market advantage. This approach is now embedded in Mabion’s standard manufacturing offerings for early- and late-stage customers.
Measuring Success – How to Track and Optimize Your Time to Market Strategy
There are many parameters by which you can predict how long it will take for a drug to enter the market. Among the most popular are Cell Line Productivity, engineering Batch Success Rate or product-specific Approval Readiness Score. Low productivity increases the number of production runs required, extending timelines for material availability. Low success rate leads to rework, investigation delays, and manufacturing slot rescheduling. A composite measure of CMC completeness, analytical readiness and quality documentation status significantly affects registration readiness. Poor documentation or disorganized teams result in prolonged agency communication cycles.
It is the time indicators that are the most popular parameters of success. For commercial scale manufacturing, Batch Cycle Time, defined as the total time from start of cell culture to end of drug substance production per batch, is crucial. Long cycles, typical for biologic drugs, reduce annual batch capacity and delay material availability.20
For drugs that are in the early stages of clinical trials, much more important metrics are time to first patient dosed, time required to finalize upstream and downstream bioprocesses before entering GMP production, or analytical method qualification lead time. A biopharmaceutical company using the CDMO platform’s CHO line completes cell line development in 4 months instead of the industry average of 9-12 months, gaining early access to material for preclinical studies.21 By using a pre-validated platform process, service providers reduces process lock time from 8 to 5 months. A vendor that pre-validates analytical methods during Phase II avoids a 6-month delay in Phase III readiness.22 But, delays in release testing or OOS investigations can stall batch release and subsequent clinical or commercial activities. That’s why it makes sense to trust a proven, reliable partner.
A sponsor who begins CMC documentation preparation alongside process development typically submits their IND 6 weeks ahead of plan. Centralized GMP fill-finish and logistics coordination enables patient dosing within 3 weeks of IND approval. When integrated into a unified project tracking system, these KPIs give both sponsors and CDMOs the ability to drive faster, more predictable market entry.
Conclusion
Biologic drug development is a race against time and the CDMO model has become one of the most effective tools to win it.23 By leveraging platform processes, integrated manufacturing systems, and pre-validated analytical methods, CDMOs offer biotech innovators a way to drastically reduce delays and increase regulatory readiness.24

For sponsors, choosing the right CDMO partner means moving faster and more confidently toward regulatory approval and commercial success. It also means an increased chance that the very drug they produce will be the first to enter the market.
Prepared by:

Bartłomiej Czubek
Director of Business Development

Jakub Knurek
Marketing Specialist
References
- Idris SA, Hussien TMA, Al-Shammari FF, Nagi HA, Bashir AI, Elhussein GEMO, Abdalla RAH, Mohammed HME, Abdelaziz WE, Alshammari AD, Alreshidi HFH, Alshammari HNM, Ibrahim SIB. An Evaluation of Drug Prescribing Patterns and Prescription Completeness. Healthcare (Basel). 2024; 12(22): 2221.
- Hardman TC, Aitchison R, Scaife R, Edwards J, Slater G. The future of clinical trials and drug development: 2050. Drugs Context. 2023; 12: 2023-2-2.
- The Association of the British Pharmaceutical Industry, Intellectual property: enabling next-generation innovation. 2025. Available at: https://www.abpi.org.uk/international-trade-and-ip/intellectual-property-ip/intellectual-property-enabling-next-generation-innovation/
- Roche, Finance Reports. Available at: https://www.roche.com/investors/reports
- Abbvie, AbbVie Reports Full-Year and Fourth-Quarter 2022 Financial Results. 2023. Available at: https://investors.abbvie.com/news-releases/news-release-details/abbvie-reports-full-year-and-fourth-quarter-2022-financial
- Merck, Merck Announces Fourth-Quarter and Full-Year 2024 Financial Results. 2025. Available at: https://www.merck.com/news/merck-announces-fourth-quarter-and-full-year-2024-financial-results/
- Ahi A, Sinkovics N, Shildibekov Y, Sinkovics R, Mehandjiev N. Advanced technologies and international business: A multidisciplinary analysis of the literature. IBR. 2022; 31(4): 101967.
- Bruker Cellular Analysis. Beacon Select™ for Cell Line Development. 2023. Available at: https://brukercellularanalysis.com/products/instruments/beacon-select-optofluidic-system/
- Cytiva. Xcellerex. 2020. Available at: https://www.cytivalifesciences.com/en/us/about-us/our-brands/xcellerex
- KrosFlo® KR2i TFF System. 2023. Available at: https://www.repligen.com/products/downstream-filtration/krosflo-tff-systems/krosflo-kr2i
- Kühner. SB2500-Z single-use bioreactor. 2022. Available at: https://kuhner.com/en/products/data/SB2500-Z.php
- Tuszyner A, Miłek P, Toboła P. Impact of glycosylation on the effector functions of monoclonal antibodies: a case study of rituximab. Mabion Science Hub. 2023. Available at: https://www.mabion.eu/science-hub/articles/impact-of-glycosylation-on-antibody-functions/
- Sampathkumar K, Kerwin BA. Roadmap for Drug Product Development and Manufacturing of Biologics. J Pharm Sci. 2024; 113(2): 314-331.
- Wines BD, Trist HM, Esparon S, Impey RE, Mackay GA, Andrews RK, Soares da Costa TP, Pietersz GA, Baker RI, Hogarth PM. Fc Binding by FcγRIIa Is Essential for Cellular Activation by the Anti-FcγRIIa mAbs 8.26 and 8.2. Front Immunol. 2021; 12: 666813.
- Tuszyner A, Durys N. Mycoplasma contamination in biopharmaceutical manufacturing. Mabion Science Hub. 2023. Available at: https://www.mabion.eu/science-hub/articles/mycoplasma-contamination-in-biologics-manufacturing/
- European Medicines Agency, ICH Q2(R2) Validation of analytical procedures
- European Medicines Agency, ICH Q6B Specifications: test procedures and acceptance criteria for biotechnological/biological products
- Stasiak A. A synergy of excellence: Novavax-Mabion partnership on vaccine during unprecedented times amid the COVID-19 pandemic. Mabion Science Hub. 2024. Available at: https://www.mabion.eu/science-hub/articles/a-synergy-of-excellence-novavax-mabion-partnership-during-unprecedented-times-amid-the-covid-19-pandemic/
- U. S. Food and Drug Administration. Investigational New Drug (IND) Application. Available at: https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application
- David L, Schwan P, Lobedann M, Borchert SO, Budde B, Temming M, Kuerschner M, Alberti Aguilo FM, Baumarth K, Thüte T, Maiser B, Blank A, Kistler V, Weber N, Brandt H, Poggel M, Kaiser K, Geisen K, Oehme F, Schembecker G. Side-by-side comparability of batch and continuous downstream for the production of monoclonal antibodies. Biotechnol Bioeng. 2020; 117(4): 1024-1036.
- Ganapathy K, Lam C, Tsukuda J, Sargon A, Nava A, Harms P, Shen A, Barnard G, Misaghi S. SPEED-MODE cell line development (CLD): Reducing Chinese hamster ovary (CHO) CLD timelines via earlier suspension adaptation and maximizing time spent in the exponential growth phase. Biotechnol Prog. 2024; 40(5): e3479.
- Małecka A, Toboła P. Does Your CDMO Have An Analytical Edge? Outsourced Pharma. 2024.
- Kulkova J, Kulkov I, Rohrbeck R, Lu S, Khwaja A, Karjaluoto H, Mero J. Medicine of the future: How and who is going to treat us? Futures. 2023; 146: 103097.
- Erickson J, Baker J, Barrett S, Brady C, Brower M, Carbonell R, Charlebois T, Coffman J, Connell-Crowley L, Coolbaugh M, Fallon E, Garr E, Gillespie C, Hart R, Haug A, Nyberg G, Phillips M, Pollard D, Qadan M, Ramos I, Rogers K, Schaefer G, Walther J, Lee K. End-to-end collaboration to transform biopharmaceutical development and manufacturing. Biotechnol Bioeng. 2021; 118(9): 3302-3312.
- Sartorius, Biopharmaceuticals: Faster to Market. 2022. Available at: https://www.sartorius.com/en/company/newsroom/blog/biopharmaceuticals-faster-to-market-1174806
Related resources

Biologics Characterization for Ensuring Product Quality and Consistency
Analytics, Biologics
Monoclonal Antibody Prophylaxis for Infectious Diseases – Passive Immunization and Prevention
Monoclonal antibody, Vaccines

End-to-End Manufacturing of Biosimilars – From Cell Line to Commercial Product
Biosimilars, Manufacturing