Rapid Microbiological Methods in Quality Control of Sterile Drugs
Contamination, Drug product, Microbiology
The production of sterile products requires the highest standards of pharmaceutical quality assurance, because even single microorganism can compromise patient safety. Traditionally, classical microbiological testing rely on cultured-based methods such as bioburden testing, sterility, and environmental monitoring. Unfortunately, traditional methods, despite being well established and accepted by pharmacopoeias, have significant limitations, mainly related to long incubation times or dependance on the growth of microorganisms in laboratory conditions. These restrictions led to the development of rapid microbiological methods (RMMs), which are based on techniques that reportedly provide accurate and reliable results of microbial contamination detection in a shorter time and often with less operator involvement.1,2
What Are Rapid Microbiological Methods in Sterile Drug Quality Control?
Rapid Microbiological Methods (RMMs) in Sterile drug Quality Control are wide group of modern rapid testing techniques designed to detect, identify, and quantify microorganisms faster and more accurately than traditional, labor-intensive Pasteurian methods. The advantages of these methods include not only reduced time and effort, but also the possibility of automation and improved parameters such as accuracy and sensitivity.
In the case of rapid microbiological methods, we can distinguish between qualitative, quantitative, or identification testing as a type of determination. Qualitative testing allows for the assessment of the presence or absence of live bacteria, quantitative testing allows for the counting of microorganisms in the test sample, while identification tests provide us with an answer related to classification into a specific taxonomic group.2
Additionally, within rapid microbiological methods, we distinguish three main detection methods, and these are:
- Growth-based detection – detects microorganisms proliferating on liquid or solid media by measuring parameters of physiological or chemical growth, but in a faster way than traditional culture.3
- Direct analysis – they are used to detect whole bacteria.
- Analysis of cell components – targets unique microbial biomolecules e.g. proteins, nucleic acids, lipids, fatty acids, etc.2
Regulatory agencies (FDA, EMA) recognize rapid microbiological methods as alternatives to conventional methods. Regulatory requirements for sterile drugs and GMP compliance necessitate validation of their usability and confirmation of their equivalence or superiority in relation to pharmacopoeial methods.3
How Do Rapid Microbiological Methods Improve Sterile Drug Testing?
The new methods offer many advantages, the main one being the ability to obtain time-to-result in drug testing in real time or within hours to a few days. Depending on the method, they can also significantly improve its detection limit, accuracy, or other key attributes related to the method.4 What is more many rapid microbiological methods systems are automated, reducing human error and operator variability and because it significantly reduces the time needed to obtain results, the implementation of these methods also has economic benefits. Under normal conditions, the production process is halted, or the product is stored until the absence of contamination has been verified. Rapid microbiological methods feature a simple-to-use protocol, minimal waste generation, lower operator variability, and reduced staff training requirements.5
Top Rapid Microbiological Techniques Used in Sterile Drug Quality Control
Adenosine Tri-Phosphate (ATP) Bioluminescence
Every living, metabolically active cell is characterized by the presence of ATP. In dead cells, cellular metabolism is disrupted, leading to a rapid decrease in intracellular ATP. In the presence of the substrate D-luciferin, oxygen, and magnesium ions, the enzyme luciferase uses energy from ATP to oxidize D-luciferin, resulting in a bioluminescent flash. The amount of light produced can be measured using luminometers, and its amount is directly proportional to the amount of ATP in the test sample.6 Using this method, it is possible to obtain results within 1 h or 4 to 7 days for sterility test.2
Autofluorescence
Autofluorescence technology exploits the fact that cells fluoresce when illuminated with blue light. It has been shown that oxidized flavins (FAD, FMN, riboflavin) are largely responsible for these signals.7 The principle of operation is based on three steps. The first is excitation, i.e., the illumination of microorganisms using light of a specific wavelength. This is followed by the emission of light with a longer wavelength by internal fluorophores, with each molecule having its own characteristic emission spectrum. Sensitive detectors capture and quantify emitted light, which, after appropriate analysis, can reveal information about the presence, quantity, and sometimes even the metabolic status of microorganisms.8 Using this method, it is possible to obtain results within 3 hours.2
Flow cytometry
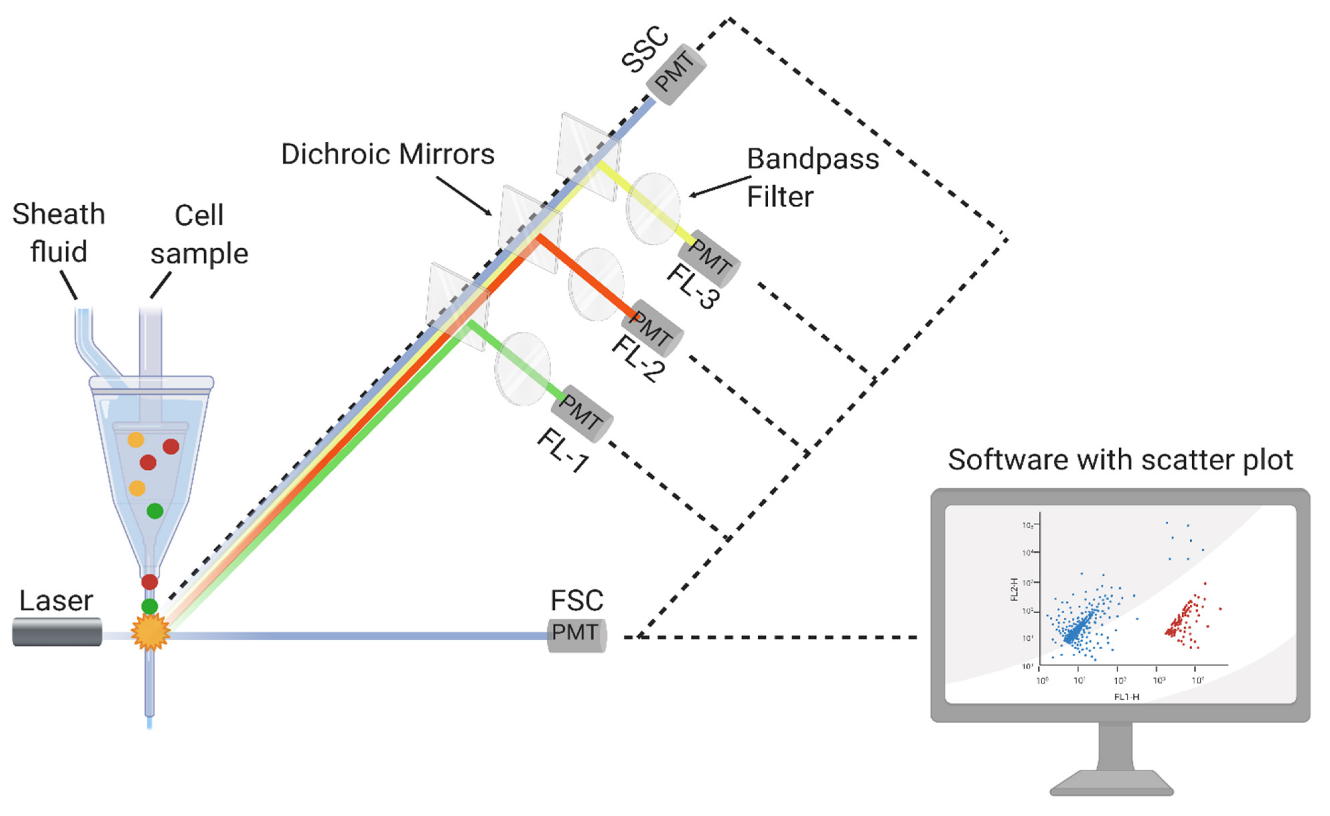
Flow cytometry is an advanced analytical technique used to evaluate individual cells in suspension. Cells passing through a laser beam are analyzed for their physical and biological properties. This technology is based on labeling microorganisms in solution with a non-fluorescent marker. This technology is based on labeling microorganisms in solution with a non-fluorescent marker, which, after being absorbed by the cell and acted upon by intracellular enzymes, is converted into a fluorescent substrate. The labeled sample is passed through a laser beam, which detects each microorganism individually. The result is usually obtained within 1.5 to 2 hours, with a detection limit of approximately 100 CFU per mL.6
Fatty Acid Profiles
Bacteria possess a very wide variety of acyl fatty acid chains. Determining the composition of fatty acid groups can be useful in identifying prokaryotic taxa at the species level. The technique involves culturing isolates on standard media, followed by fatty acid saponification, methylation, and extraction. These processes result in the formation of fatty acid methyl esters (FAME). FAME are then evaluated using gas chromatography (GC), where the molecules undergo ionization and the masses of these ions in the gas phase are determined. The retention times of peaks from test samples are compared with the retention times of known standards.10 Using this method, it is possible to obtain results within 24-48 hours.2
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR technique is based on the principle of absorption by molecules of specific frequencies of infrared light corresponding to the frequency of vibrations of their chemical bonds. This absorption leads to the formation of a characteristic spectrum that allows the identification of molecular structures and functional groups present in the sample. This spectrum is a kind of fingerprint that can be used for qualitative and quantitative analysis. FTIR can be used to examine a wide range of samples, including solids, liquids, and gases. In addition, spectroscopy-based techniques require minimal sample preparation, i.e., they do not require any labeling, staining, amplification, or extraction. In addition, only a small amount of bacterial biomass is needed to obtain spectra, and results can be obtained within 6-8 hours.11,12

Why Speed Matters? – The Importance of Rapid Testing in Sterile Drug Production
The ultimate goal of sterility testing in drug manufacturing is patient protection and safety. Unfortunately, using traditional testing methods, contamination can only be detected no earlier than 14 days after the product is manufactured. What is more, many sterile products have short shelf lives. During testing, the product is in a state of suspension, and waiting two weeks for the results reduces its usability. Furthermore, holding back the product while awaiting results may lead to increased storage costs and slow down the supply chain. With shorter analysis times, they facilitate testing and reduce the probability of contamination, positively impacting reliability. This can improve production in-process testing, quality control of biologics time, increase safety and enable faster implementation of any corrective actions.2,3,15
Strategy for implementing Rapid Microbiological Methods
The strategy for implementing rapid microbiological methods should be based on due diligence analysis, considering both scientific and business perspectives. When planning, consideration should be given to selecting the appropriate technology platform, developing a detailed implementation strategy, and providing sound justification for validation and regulatory plans. Developing the necessary justifications for introducing new rapid microbiological methods essentially involves three stages:
- Review of existing conventional methods and verification of the qualitative, technological, and business possibilities of replacing them with RMMs.
- Identifying the available RMMs technologies that can meet the business and technological needs of the company.
- Preparation of an economic justification for replacing the conventional method with RMMs.
After conducting due diligence and making a positive decision to continue the work, proceed to develop a plan for validating and regulating the method, as well as a plan for its implementation.14
Challenges and Limitations of Rapid Microbiological Methods in Sterile Drug Testing
The implementation of rapid microbiological methods in the pharmaceutical industry and sterile drug testing is associated with several challenges and limitations. Among them, we highlight the need for high technical knowledge, experience related to operation, installation and operating costs of the method, as well as validation and regulatory support. A risk analysis for the introduction of such a method is also required, and the validation process will include detailed testing stages for each product to confirm the suitability of the selected RMMs method, given the lack of a universal method applicable to the testing of all types of pharmaceutical products. In addition, regulatory control is necessary to ensure the reproducibility and accuracy of the results obtained, but both the European Pharmacopoeia and the USP encourage the implementation and use of RMMs. In addition, regulatory control is necessary to ensure the reproducibility and accuracy of the results obtained. However, both the European Pharmacopoeia and the USP encourage the implementation and use of rapid microbiological methods, which, under an appropriate risk control strategy, can provide a highly sensitive, rapid, and accurate verification system ensuring product sterility and, consequently, the most important aspect: patient safety.15

FAQ
Prepared by:

Karolina Kromkowska
Microbiology Specialist
References
- Peris-Vicente J, Carda-Broch S, Esteve-Romero J. Validation of Rapid Microbiological Methods. Journal of Laboratory Automation. 2014; 20(3): 259-264.
- Henriques J, Cardoso C, Vitorino C. Rapid microbiological methods. They are rapid! Are they fast? MedDocs Publishers LLC. 2019. Reno, NV.
- Marsit NM, Saadawi S, Alennabi K. Challenges of growth-based microbiological methods in sterility assurance of pharmaceutical product manufacturing. Discov. Pharm. Sci. 2025; 1: 13.
- Moldenhauer J. Validation of Rapid Microbiological Methods (RMMs). [In:] Kolhe, P., Shah, M., Rathore, N. (eds) Sterile Product Development. AAPS Advances in the Pharmaceutical Sciences Series, vol 6. Springer, 2013. New York, NY.
- Peris-Vicente J, Carda-Broch S, Esteve-Romero J. Validation of Rapid Microbiological Methods. Journal of Laboratory Automation. 2014; 20(3): 259-264.
- Moldenhauer, J. Rapid microbiological methods and the PAT initiative. BioPharm International, 2005; 18(12): 31-46.
- London R, Schwedock J, Sage A, Valley H, Meadows J, et al. An Automated System for Rapid Non-Destructive Enumeration of Growing Microbes. PLOS ONE. 2010; 5: e8609.
- Sun, X., Wang, Y., Du, A. et al. Autofluorescence properties of wound-associated bacteria cultured under various temperature, salinity, and pH conditions. BMC Microbiol. 2025; 25: 511.
- Zand E, Froehling A, Schoenher C, Zunabovic-Pichler M, Schlueter O, Jaeger H. Potential of Flow Cytometric Approaches for Rapid Microbial Detection and Characterization in the Food Industry—A Review. Foods. 2021; 10(12): 3112.
- Milton S. da Costa, Luciana Albuquerque, M. Fernanda Nobre, Robin Wait, 8 – The Identification of Fatty Acids in Bacteria, Methods in Microbiology. Academic Press. 2011; 38: 183-196.
- Kassem A, Abbas L, Coutinho O, Opara S, Najaf H, Kasperek D, Pokhrel K, Li X, Tiquia-Arashiro S. Applications of Fourier Transform-Infrared spectroscopy in microbial cell biology and environmental microbiology: advances, challenges, and future perspectives. Front Microbiol. 2023; 14: 1304081.
- Maquelin Kirschner C, Choo-Smith LNgo-Thi NA, van Vreeswijk T, Stämmler M, Endtz HP, Bruining HA, Naumann D, Puppels GJ. Prospective Study of the Performance of Vibrational Spectroscopies for Rapid Identification of Bacterial and Fungal Pathogens Recovered from Blood Cultures. J Clin Microbiol. 2003: 41(1): 324-9.
- Xu J-L, Herrero-Langreo A, Lamba S, Ferone M, Scannell AGM, Caponigro V, Gowen AA. Characterisation and Classification of Foodborne Bacteria Using Reflectance FTIR Microscopic Imaging. Molecules, 26(20), 6318.
- Miller MJ. The implementation of rapid microbiological methods. European Pharmaceutical Review 2010; 15(6): 27-31.
- Marsit NM, Saadawi S, Alennabi K. Challenges of growth-based microbiological methods in sterility assurance of pharmaceutical product manufacturing. Discov. Pharm. Sci. 2025; 1: 13.
Related resources

From Research to Release: Mapping Mabion’s Full Analytical Lifecycle for Monoclonal Antibodies
Analytics, Mabion, Monoclonal antibody

The New Era of Biosimilar Development: Seizing the Opportunity Under EMA’s Streamlined Guidelines
Biosimilars, Clinical trials, Drug development, EMA, FDA, Mabion, Regulatory

The Role of Downstream Development in Ensuring Biologic Product Purity and Quality
Drug product, Manufacturing