Monoclonal Antibody Prophylaxis for Infectious Diseases – Passive Immunization and Prevention
Monoclonal antibody, Vaccines
This article reviews how monoclonal antibody prophylaxis for infectious diseases complements vaccines through passive immunization with monoclonal antibodies. Infectious diseases remain among the leading causes of morbidity and mortality worldwide, despite significant progress in prevention and treatment strategies. Traditional approaches have reshaped public health, drastically lowering the burden of diseases such as smallpox, poliomyelitis, and rotavirus diarrhea. Yet, even with these interventions, substantial gaps remain: no effective vaccines exist for several major pathogens, antimicrobial resistance is on the rise, and novel threats such as SARS-CoV-2 continue to challenge global health systems.
In this context, monoclonal antibodies have emerged as a transformative tool in infectious disease prevention. These biologic therapies, once largely confined to oncology and autoimmune disorders, now offer targeted, immediate protection against infectious agents.
Standard infectious disease prevention and where monoclonal antibody prophylaxis fits
Infectious diseases have historically been controlled through a combination of public health measures, hygiene practices, vaccination, and antimicrobial treatments. Improvements in sanitation, clean water, and awareness of microbes in the environment have dramatically reduced the burden of infections such as diarrheal illnesses.1 For example, investments in water, sanitation and hygiene (WASH) infrastructure during 1990-2015 halved global deaths from diarrheal disease. Simple practices like regular hand washing, safe food handling, and maintaining clean environments interrupt the transmission of pathogens and are fundamental to disease prevention.2
Vaccination is a cornerstone of infectious disease prevention. Vaccines prime the immune system against specific pathogens, dramatically reducing the incidence of many life-threatening diseases. The introduction of routine immunization programs over the 20th century led to the global eradication of smallpox3 and the regional elimination of polio,4 among other successes. Widespread vaccination against childhood infections like measles, diphtheria, and pertussis has saved countless lives and in some cases eliminated these diseases from entire populations. For instance, vaccines against rotavirus and hepatitis B (both protein-based vaccines) have markedly reduced disease burden: rotavirus immunization has been associated with an ~80% drop in hospitalizations for severe infant diarrhea, and hepatitis B vaccination (using the viral surface protein as antigen) provides nearly 100% protection against HBV infection, preventing transmission and subsequent liver cancer. These examples illustrate the central role of vaccines in preventing infections before they occur, often conferring long-term immunity and even herd immunity when coverage is high. Indeed, by conferring community-wide protection, high vaccination coverage protects vulnerable individuals who cannot be vaccinated, an effect known as herd immunity.5-9
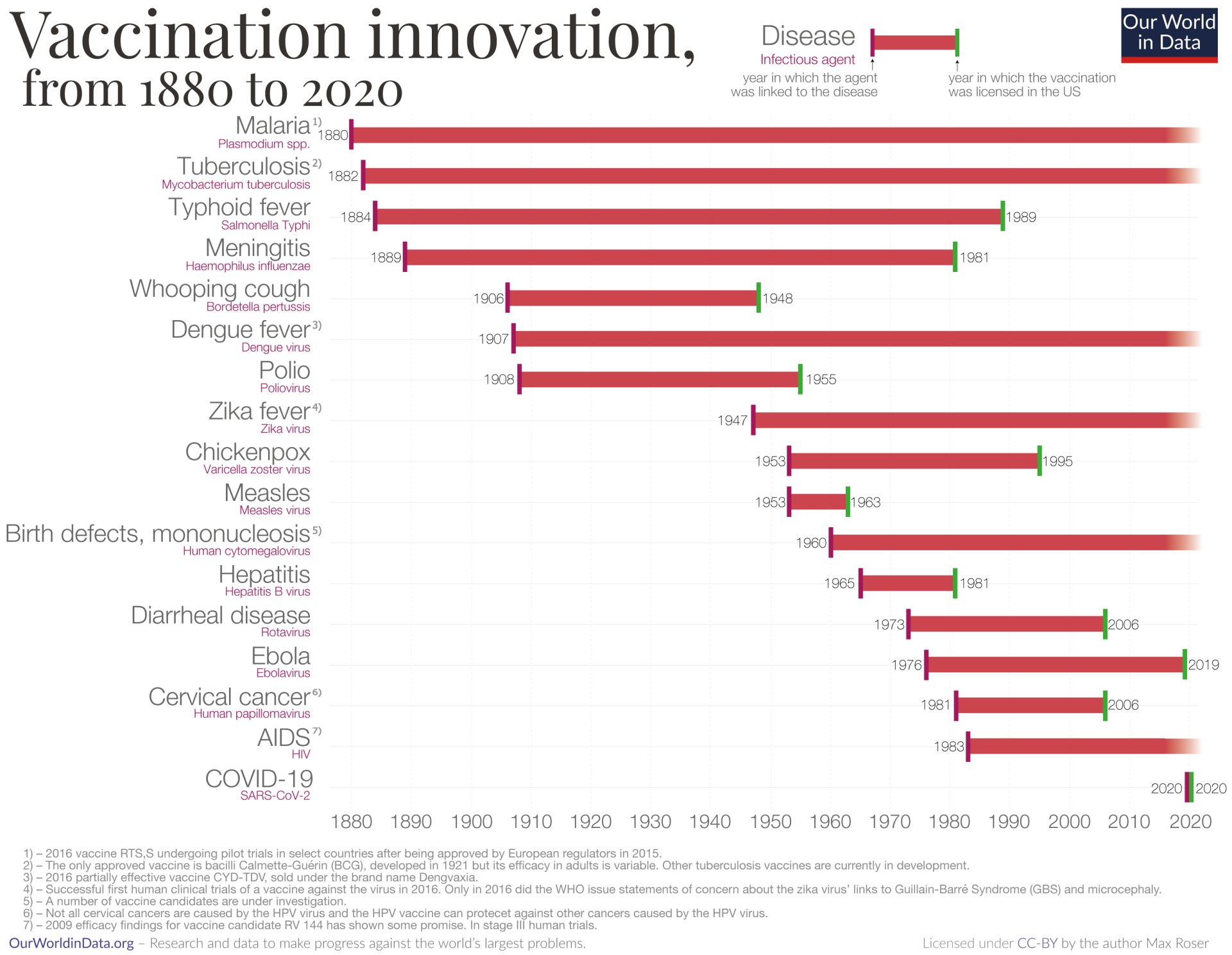
When infections do occur, antibiotics and antivirals serve as the first line of therapy to control and treat disease. Antibiotic drugs revolutionized medicine in the 20th century by curing bacterial infections that were once frequently fatal.10 These drugs work by killing bacteria or halting their growth, aiding the body’s immune defenses in clearing the infection. Similarly, antiviral medications can suppress or shorten the course of certain viral illnesses. The availability of antimicrobial therapy means that many infections that cannot be prevented can at least be treated effectively, reducing complications and mortality.
How do vaccines provide immunity and protect against infectious diseases?
Vaccines protect against infectious diseases by inducing active immunity. They train the body’s immune system to recognize and combat specific pathogens. When a person is vaccinated, a harmless form or component of a microbe (the antigen) is introduced, mimicking an infection just enough to stimulate the immune defenses. The vaccine antigens may consist of weakened live microbes, inactivated (killed) organisms, purified proteins (subunit vaccines), or pieces of genetic material, as well as toxoids (inactivated toxins) for bacterial diseases like tetanus. This exposure triggers the body’s adaptive immune response: specialized white blood cells recognize the foreign antigen and become activated. B cells produce specific antibodies that bind to the pathogen, while T cells help destroy infected cells or assist B cells. Through this process, vaccination prompts the immune system to “learn” the pathogen’s identity without causing the actual disease. This natural mechanism illustrates the fundamentals of antibodies development in the immune response. The immediate result is the generation of effector immune cells and antibodies that can neutralize the invader.11
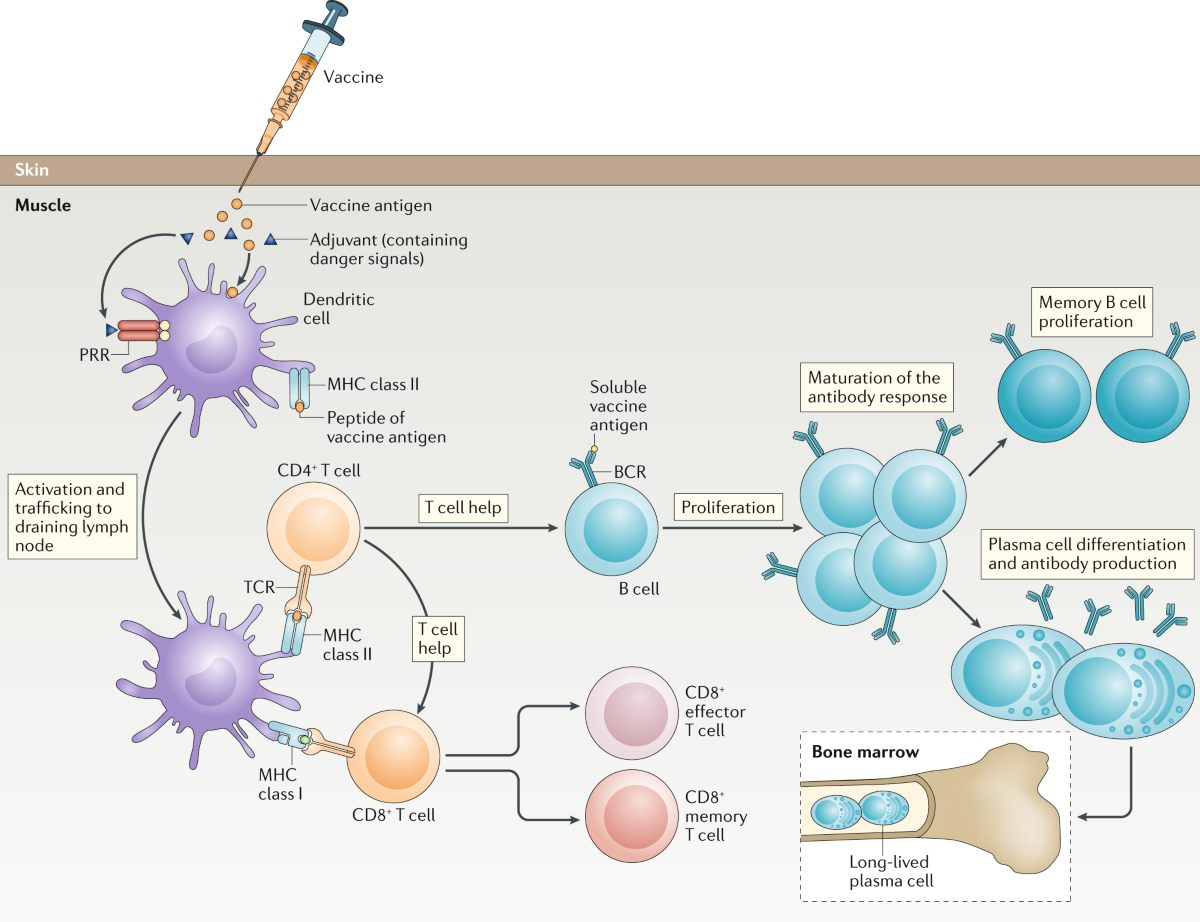
A crucial feature of vaccines is the creation of immunological memory. After the initial immune response to a vaccine, a subset of B and T lymphocytes persists as long-lived memory cells. These cells remain vigilant in the body, often for years or decades, even after antibody levels from the vaccine have waned. If the real pathogen is encountered in the future, the immune system is able to recall its prior training and respond much more rapidly and effectively. Memory B cells quickly proliferate and produce high levels of antibodies, while memory T cells swiftly coordinate an attack on infected cells. In this way, vaccines establish a state of preparedness that can prevent an infection from taking hold or at least mitigate its severity.12,13 For instance, the hepatitis B vaccine induces enduring antibody-mediated immunity such that exposure to hepatitis B virus is neutralized before it can cause illness. Many vaccines, like those for measles or polio, confer long-lasting or lifelong immunity in most recipients by virtue of robust memory cell generation.
The protection afforded by vaccines operates at both the individual and population level. On an individual level, an effective vaccine drastically lowers the risk of disease upon exposure – a vaccinated person’s immune system can eliminate the pathogen so quickly that they either do not get infected or only experience a very mild illness. Even in cases where vaccination does not completely prevent infection, it typically blunts the severity of disease. A primed immune system can keep pathogen levels low and prevent complications, so vaccinated individuals are far less likely to suffer severe outcomes or death compared to the unvaccinated. This has been observed with many vaccines; for example, influenza and COVID-19 vaccines may not always block mild infections, but they are highly effective at preventing hospitalization and fatal disease in those who do get infected.14
At the community level, widespread vaccination can achieve herd immunity, wherein transmission chains are disrupted because so many people are immune that the pathogen struggles to find new susceptible hosts. This indirect protection is critical for safeguarding those who cannot be vaccinated (such as infants too young for certain vaccines or individuals with contraindications). In effect, vaccines reduce the overall amount of pathogen circulating in a population, thereby protecting the community as a whole.15
There are several categories of vaccines, and they provide immunity in slightly different ways. Live-attenuated vaccines (e.g. measles, mumps, rubella, or the varicella chickenpox vaccine) contain a weakened form of the pathogen that can replicate to a limited extent without causing disease. These tend to elicit strong, long-lasting immune responses often with one or two doses, closely mimicking natural infection and inducing both antibody and T-cell memory. By contrast, inactivated vaccines and subunit (protein) vaccines (such as the injectable polio vaccine, hepatitis B vaccine, or many influenza vaccines) contain no live agent. They typically require multiple doses plus occasional boosters to maintain immunity, because the immune response they induce can wane over time.16 For example, the immunity from acellular pertussis vaccines or influenza vaccines diminishes within a couple of years, necessitating booster shots or annual re-vaccination. However, these non-live vaccines are very safe even in people with weakened immune systems. Additionally, adjuvants are often included in such vaccines to enhance the immune response. Despite differences in dosing and duration, all vaccine types aim to establish a pool of memory lymphocytes and circulating antibodies specific to the pathogen of interest.17
Finally, it is important to note that vaccination is far safer than contracting the infection itself. The transient side effects of vaccines (such as low-grade fever or soreness) pale in comparison to the potentially severe and unpredictable complications of natural infection. By pre-arming the immune system, vaccines avert the high stakes of a first encounter with virulent pathogens. The dramatic global declines in diseases like Haemophilus influenzae type b meningitis, rotavirus diarrhea, and cervical cancer (linked to HPV) following vaccine introduction are testaments to the power of this preventive approach. Vaccines provide immunity by simulating infection in a controlled way, thereby provoking a protective immune memory that shields individuals and communities from future disease.18
Limitations of vaccines and gaps addressed by passive immunization with monoclonal antibodies
While vaccines have proven enormously effective, there are notable limitations to vaccine-based protection that leave gaps in our defenses. First, for many infectious diseases, no effective vaccine exists at all. Despite decades of research, scientists have been unable to develop vaccines for challenging pathogens such as HIV (the virus that causes AIDS) or malaria (caused by Plasmodium parasites). In both cases, the studies are still ongoing.19–21
The traditional vaccine paradigm – as pioneered by Jenner and Pasteur – has struggled against these complex foes due to several factors: extraordinary genetic variability of the pathogen, sophisticated immune evasion strategies, and in the case of malaria, a multi-stage lifecycle. HIV, for example, exists as countless evolving strains and directly attacks the immune system’s T cells, thwarting efforts to induce broadly protective immunity.20 Similarly, the hepatitis C virus (HCV) rapidly mutates and has defied vaccine development. In addition, certain bacterial infections like Staphylococcus aureus or E. coli have not yielded a successful vaccine, and emerging threats (e.g. Zika virus or pathogenic E. coli strains) often face long vaccine development timelines. Even some historically devastating diseases still lack a universally efficacious vaccine.
Tuberculosis (TB) is a prime example – the only licensed vaccine, BCG, was developed over a century ago (1921) and provides very limited protection, primarily working in infants and young children. It does not reliably protect adolescents or adults against pulmonary TB, which is the contagious form of the disease. Consequently, TB remains one of the top infectious killers worldwide, illustrating the tragic consequences when vaccination tools are inadequate or nonexistent.22 Fortunately, clinical trials of more effective tuberculosis vaccine candidates are underway.23
Even when vaccines do exist, partial or suboptimal efficacy can limit their protective impact. No vaccine is 100% effective in every individual or against every strain of a pathogen. Many vaccines significantly reduce the risk of disease but do not guarantee complete immunity. For instance, the seasonal influenza vaccine must be updated annually to match circulating virus strains, and its efficacy varies year to year. In healthy adults under 65, flu vaccine efficacy averages around ~59% in preventing clinical illness, but in some mismatched seasons efficacy can be substantially lower. Influenza viruses constantly mutate their surface antigens (a phenomenon known as antigenic drift), so a vaccine formulated months in advance may be less effective if the virus changes.24
Some vaccines primarily prevent severe disease but do not always block infection or transmission (for example, COVID-19 vaccines are highly protective against hospitalization but cannot completely stop mild breakthrough infections by new variants). These efficacy limitations mean that even vaccinated individuals might occasionally fall ill, and achieving herd immunity may be difficult due to anti-vaccination sentiment among the public.25
Another challenge is that pathogen diversity and evolution can outpace vaccine protection. RNA viruses like influenza and HIV mutate rapidly; new variants or serotypes can emerge that partially escape the immunity induced by existing vaccines.26 We saw this during the COVID-19 pandemic: the SARS-CoV-2 virus evolved variants of concern with mutations in the spike protein that reduced vaccine-induced protection and necessitated booster doses and updated vaccine formulations. Similarly, the Omicron variant’s many spike mutations allowed it to evade a degree of the neutralizing antibodies generated by vaccines or prior infection, leading to more breakthrough cases. Vaccine strategies must continuously adapt. For influenza, this means annual reformulation of the vaccine cocktail, and for COVID-19, bivalent boosters were introduced targeting newer variants.27
Vaccines also face practical and population-level limitations. Not everyone can be safely vaccinated. Individuals with certain medical conditions or severe immunodeficiencies may be unable to receive live attenuated vaccines due to risk of disease from the vaccine strain. For example, people undergoing chemotherapy or living with advanced HIV infection cannot get live vaccines like the yellow fever or varicella vaccines, leaving them reliant on herd immunity or alternative prophylaxis.28 Even in healthy people, immune responses to vaccines can vary: the elderly often mount weaker responses (hence special high-dose flu vaccines are formulated for seniors), and some individuals are “non-responders” to certain vaccines for genetic reasons (a small percentage might not produce antibodies after hepatitis B vaccination, for instance). Additionally, most vaccines require a series of doses for full protection – if those doses are not completed or boosters not administered on schedule, immunity may be insufficient. This raises issues of compliance and access, especially in resource-limited settings. Keeping immunity up to date (through booster shots for tetanus every 10 years, or periodic boosters for COVID-19) is logistically challenging on a population scale. Moreover, cold chain requirements for vaccine storage and distribution can impede vaccination efforts in remote or undeveloped regions. Vaccines like the mRNA COVID-19 vaccines initially required ultra-cold storage, which was a hurdle for low-income countries. Such practical barriers mean that even where efficacious vaccines exist, achieving high coverage can be difficult. Gaps in coverage, in turn, allow outbreaks of vaccine-preventable diseases to occur (as seen in recent measles outbreaks when vaccination rates fell below herd immunity thresholds).29
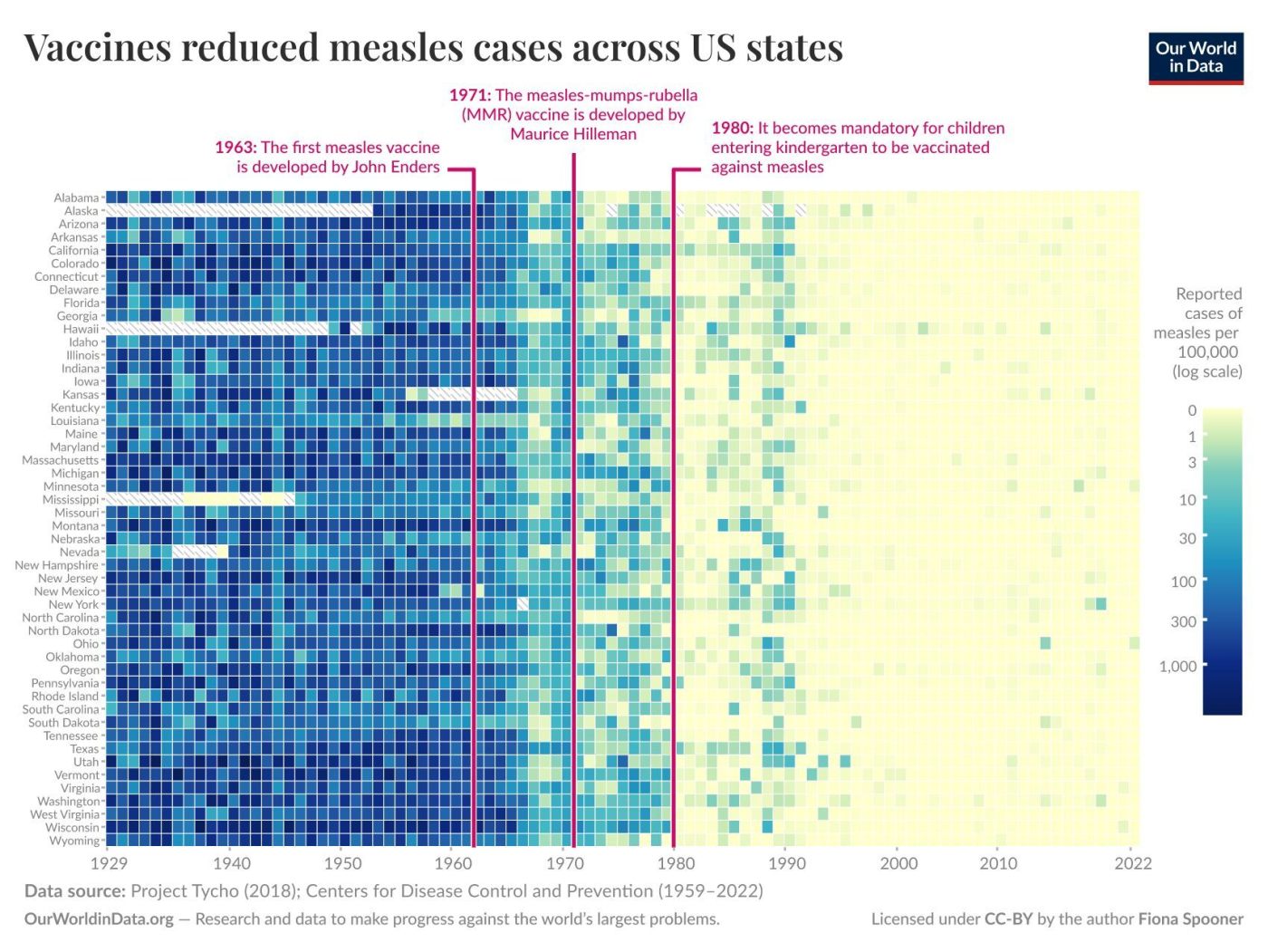
Finally, vaccine development is a slow, costly process, which limits our ability to respond quickly to emerging threats. It often takes many years and substantial financial investment to research, test, and approve a new vaccine. While the COVID-19 vaccines were developed in record time (about 11 months from genomic sequence to authorization, an unprecedented feat), this is the exception rather than the norm.31 Many diseases (e.g. EBV, RSV) have had vaccine candidates in research for decades without a licensed product yet.32,33 During the years (or decades) while we lack a vaccine, those diseases continue to cause illness and death. Even after a vaccine is developed, manufacturing and distributing millions of doses worldwide is a monumental challenge, as the pandemic revealed. Inequities in vaccine access mean that solely relying on vaccines can leave large portions of the global population unprotected for extended periods. In summary, vaccines are invaluable but not infallible. The limitations create an impetus to explore complementary approaches. This is where recent advances in immunotherapy, particularly monoclonal antibody prophylaxis, are stepping in to bolster our armamentarium against infectious diseases.
Recent advances in monoclonal antibodies for infectious disease prevention and prophylaxis
In light of the gaps left by traditional methods, recent years have witnessed major advances in immunotherapy aimed at preventing and treating infectious diseases. Central to these innovations is the use of monoclonal antibodies (mAbs) – laboratory-engineered antibodies that target specific pathogen components. The concept of using antibodies to fight infections is not new; passive immunization via antibody-rich serum was practiced over a century ago (for example, antidiphtheria serum saved children’s lives in the 1890s). Throughout the 20th century, giving patients exogenous antibodies (from human or animal donors) was found to prevent or attenuate diseases like measles, hepatitis B, rabies, and tetanus. However, traditional serum therapy and pooled immunoglobulin have limitations. They contain a mixture of many antibodies (not all of which are useful), carry risk of blood-borne pathogen transmission, and can vary by batch. The breakthrough came in the 1970s with hybridoma technology, enabling scientists to produce large quantities of a single, defined antibody with chosen specificity. Monoclonal antibodies can be made to recognize a critical antigen on a virus or bacterium with high precision. In this context, monoclonal antibodies for infectious disease prevention provide targeted, rapid protection as part of antibody-based prophylaxis.
Monoclonal antibody prophylaxis and therapy for infections have progressed remarkably in the past decade. Until recently, only a handful of anti-infective mAbs were available. The first was Palivizumab to prevent respiratory syncytial virus (RSV) infection in high-risk infants.35
By 2014, just two infectious disease mAbs were on the market. But by 2023, that landscape had changed dramatically. Over 10 monoclonal antibodies targeting infectious agents have been licensed or granted emergency use authorization.36-40 The COVID-19 pandemic was a major catalyst for this surge in mAb development. As SARS-CoV-2 spread globally in 2020, unprecedented resources were poured into isolating potent neutralizing antibodies against the virus. More than 20 anti-SARS-CoV-2 mAbs entered clinical trials in 2020–2021, and several received emergency authorizations for treating or preventing COVID-19. This rapid progress demonstrated the agility of modern antibody discovery platforms. Technologies such as B-cell cloning from convalescent patients, phage display libraries, and structure-guided antibody design have enabled researchers to identify human antibodies against new pathogens within weeks to months. By late 2021, multiple COVID-19 mAbs (e.g., bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab) were available to reduce the risk of severe disease, and one combination (tixagevimab/cilgavimab, brand name Evusheld) was authorized for prophylaxis in immunocompromised individuals. The effort against COVID-19 also advanced generalizable methods for antibody optimization, benefiting the whole field of infectious disease immunotherapy. Indeed, the knowledge gained and pipeline built for SARS-CoV-2 are now being applied to other pathogens.41,42
Beyond COVID-19, several new monoclonal antibodies have recently been approved for infectious diseases. A notable example is nirsevimab, a long-acting antibody against RSV. In 2023, nirsevimab was approved for routine use in infants as a universal RSV preventive, after trials showed it could reduce RSV hospitalizations in infants by approximately 80%. This next-generation mAb was engineered (with an Fc modification) to have an extended half-life, such that a single intramuscular dose given before the RSV season can protect an infant for about five months. Nirsevimab represents a new paradigm of “passive vaccination,” effectively providing immunity to babies too young to respond to an active vaccine.43
Other pathogen-specific mAbs have also come to market: for example, bezlotoxumab is a monoclonal antibody that binds Clostridioides difficile toxin B and is used to prevent recurrent C. difficile infection in adults.44 Raxibacumab and obiltoxaximab are monoclonals licensed for prophylaxis or treatment of inhalation anthrax.45,46 In 2020, a cocktail of three monoclonal antibodies (Inmazeb) was approved for Ebola virus disease, dramatically improving survival in Ebola patients. This is a triumph for antibody therapy in a filovirus infection.47 Meanwhile, clinical trials of monoclonals for prevention of HIV and malaria are underway. Broadly neutralizing antibodies that target conserved sites on HIV have been tested in high-risk individuals to see if periodic infusions can prevent acquisition of HIV. Initial results showed some reduction in infection risk, although not enough for widespread use unless combined with other antibodies.48 For malaria, a recent Phase II trial of an antibody (CIS43LS) against the Plasmodium falciparum circumsporozoite protein demonstrated >80% protection from infection over several months, suggesting that a single infusion before the malaria season could protect individuals in endemic areas.49
Monoclonal antibodies are now an expanding class of anti-infectives with several products in clinical or commercial use and many more in the pipeline. By providing immunity, they fill critical gaps: protecting those who cannot respond to vaccines, and countering pathogens for which vaccines don’t exist.50
Limitations of monoclonal antibodies in prophylaxis and passive immunization
Monoclonal antibodies have opened new possibilities for infectious disease prevention, but they too come with limitations that must be acknowledged. One major limitation is that passive antibody protection is temporary. Unlike vaccines, which induce the body’s own immune memory, administered antibodies circulate for only a finite time (measured by their half-life) and then degrade. Monoclonal antibodies do not elicit the host’s immune system to produce any lasting response; they provide immediate immunity but no immunological memory. As a result, the protection conferred by an antibody dose wanes as the antibody is cleared from the body, typically over a few weeks to a few months depending on the antibody’s half-life.51
Typical half-life of unmodified IgG antibodies is around 3 weeks, so a single infusion might protect for 1-3 months. Extended half-life mAbs like nirsevimab (for RSV) stretch this to about 5-6 months, but still eventually levels fall and the individual becomes susceptible again. In contrast, a vaccine might confer protection that lasts for years via immune memory cells. This means that for chronic prevention, monoclonal antibodies would require repeated administrations (e.g. an injection every season or even every month for some rapidly waning antibodies), which is not practical for large populations. The lack of durable immunity makes monoclonals more suitable for short-term protection in high-risk periods (such as providing prophylaxis during a newborn’s first RSV season, or giving travelers an antibody before a trip to a malaria-endemic area) rather than lifelong immunity. Essentially, antibodies “borrow” immunity for a while but do not train the immune system for the future.52
Monoclonal antibody prevention can also be undermined by pathogen evolution and escape mutants, similar to vaccine limitations but in some ways more acute. A monoclonal antibody is a single, precise lock-and-key match to a pathogen epitope. If the pathogen mutates that epitope, the antibody may lose effectiveness entirely. We saw this during the COVID-19 pandemic: several monoclonal antibodies that were effective against early SARS-CoV-2 strains became ineffective against new variants like Omicron, which carried mutations in the spike protein’s receptor-binding domain (the very target of those mAbs). Even antibody cocktails (combinations of two mAbs) were evaded by highly mutated variants.38 This phenomenon is a reminder that pathogens can rapidly select for escape under the pressure of a single specific antibody. Vaccines induce a polyclonal response (multiple antibody types to various epitopes, plus T-cell responses), which makes it harder for a microbe to escape all immunity at once; by contrast, a one monoclonal targets one epitope, creating a narrower selective pressure. For this reason, monoclonal therapies for viruses often employ a cocktail of two or more mAbs targeting non-overlapping epitopes, to reduce the likelihood that the virus can mutate to escape all components at once. Still, the risk remains that an antibody developed today could be rendered obsolete by a new strain tomorrow. This is especially relevant for rapidly evolving viruses (HIV, influenza, SARS-CoV-2) and for monoclonals developed against a specific strain (like an Ebola virus from one outbreak may differ from the next outbreak’s strain).53
Lastly, there are immunological considerations such as the possibility of antigenic interference and host reactions. Repeated administration of monoclonal antibodies could, in some cases, lead the patient’s immune system to recognize the therapeutic antibody as foreign and mount an anti-drug antibody response (especially if the mAb is not fully human or if given long-term). This is generally rare with fully human antibodies but can occur and would reduce the efficacy of the monoclonal or cause allergic reactions.54
Conclusion
As a strategy for infectious disease prevention, monoclonal antibody prophylaxis offers immediate, passive immunization that complements vaccines and antimicrobials. While vaccines remain the most effective and scalable means of inducing long-term immunity, monoclonal antibodies provide immediate protection and fill critical gaps for individuals who cannot be adequately protected by vaccination. Their rapid development and recent approvals for RSV prevention illustrate their expanding role in public health. However, cost, limited durability, and pathogen evolution continue to constrain their broader use. Future progress will depend on improving antibody engineering, reducing production costs, and integrating monoclonal antibodies into comprehensive prevention strategies alongside vaccines and antimicrobials. The strategic deployment of monoclonal antibodies, in combination with vaccines and antimicrobial therapies, may ultimately provide a more resilient and adaptive approach to controlling infectious threats worldwide.
FAQ
Prepared by:

Jakub Knurek
Marketing Specialist
References
- Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J Infect Dis. 2017; 215(11): 1666-1672.
- World Health Organization. Water, sanitation and hygiene (WASH).
- Strassburg MA. The global eradication of smallpox. Am J Infect Control. 1982; 10(2): 53-59.
- World Health Organization. Global Polio Eradication Initiative.
- UNICEF. Vaccines and the diseases they prevent.
- U.S. Centers for Disease Control and Prevention. Vaccines & Immunizations.
- World Health Organization. Immunization, Vaccines and Biologicals.
- European Centre for Disease Prevention and Control. Vaccine-preventable diseases.
- Spooner F, Dattani S, Vanderslott S, Roser M. Vaccination. 2022. OurWorldinData.org
- Rosen W. Miracle Cure: The Creation of Antibiotics and the Birth of Modern Medicine. Viking: New York, NY. 2017.
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021; 21(2): 83-100. Erratum in: Nat Rev Immunol. 2021; 21(2): 129.
- Bugya Z, Prechl J, Szénási T, Nemes É, Bácsi A, Koncz G. Multiple Levels of Immunological Memory and Their Association with Vaccination. Vaccines (Basel). 2021; 9(2): 174.
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010; 33(4): 451-463.
- Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022; 310(1): 27-46.
- Bullen M, Heriot GS, Jamrozik E. Herd immunity, vaccination and moral obligation. J Med Ethics. 2023; 49(9): 636-641.
- Ghattas M, Dwivedi G, Lavertu M, Alameh MG. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines (Basel). 2021; 9(12): 1490.
- Jones-Gray E, Robinson EJ, Kucharski AJ, Fox A, Sullivan SG. Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis. Lancet Respir Med. 2023; 11(1): 27-44.
- Hyde TB, Dentz H, Wang SA, Burchett HE, Mounier-Jack S, Mantel CF; New Vaccine Introduction Impact Published Literature Working Group. The impact of new vaccine introduction on immunization and health systems: a review of the published literature. Vaccine. 2012; 30(45): 6347-6358.
- Benn CS, Fisker AB, Rieckmann A, Sørup S, Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis. 2020; 20(10): e274-e283.
- Van Regenmortel MH. Editorial: Paradigm Changes are Required in HIV Vaccine Research. Front Immunol. 2015; 6: 326.
- Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010; 120(12): 4168-78.
- Zhuang L, Ye Z, Li L, Yang L, Gong W. Next-Generation TB Vaccines: Progress, Challenges, and Prospects. Vaccines (Basel). 2023; 11(8): 1304.
- Del Pozo-Ramos L, Kupz A. A review of the efficacy of clinical tuberculosis vaccine candidates in mouse models. Front Immunol. 2025; 16: 1609136.
- Maurer DP, Vu M, Schmidt AG. Antigenic drift expands influenza viral escape pathways from recalled humoral immunity. Immunity. 2025; 58(3): 716-727.
- Swan DA, Bracis C, Janes H, Moore M, Matrajt L, Reeves DB, Burns E, Donnell D, Cohen MS, Schiffer JT, Dimitrov D. COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. Sci Rep. 2021; 11(1): 15531.
- Sanjuán R, Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016; 73(23): 4433-4448.
- Kim S, Nguyen TT, Taitt AS, Jhun H, Park HY, Kim SH, Kim YG, Song EY, Lee Y, Yum H, Shin KC, Choi YK, Song CS, Yeom SC, Kim B, Netea M, Kim S. SARS-CoV-2 Omicron Mutation Is Faster than the Chase: Multiple Mutations on Spike/ACE2 Interaction Residues. Immune Netw. 2021; 21(6): e38.
- Einarsson J, Wilkinson AN. Vaccination considerations for patients receiving cancer therapy. Can Fam Physician. 2022; 68(10): 751-752.
- National Academies of Sciences, Engineering, and Medicine. Evidence review of the adverse effects of COVID-19 vaccination and intramuscular vaccine administration. The National Academies Press: Washington, DC. 2024.
- DeBold T, Friedman D. Battling Infectious Diseases in the 20th Century: The Impact of Vaccines. The Wall Street Journal. 2015.
- Wong JC, Lao CT, Yousif MM, Luga JM. Fast Tracking-Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review. Vaccines (Basel). 2022; 10(8): 1256.
- Dai Y, Zhang B, Yang L, Tao S, Yu Y, Li C. Recent Progress in the Vaccine Development Against Epstein-Barr Virus. Viruses. 2025; 17(7): 936.
- Kelleher K, Subramaniam N, Drysdale SB. The recent landscape of RSV vaccine research. Ther Adv Vaccines Immunother. 2025; 13: 25151355241310601.
- Adedokun AI, Adigun OA, Ibrahim AM, Idris I, Ntasin PY, Olowu BI, et al. Exploring immunotherapeutic strategies for bacterial and viral diseases. Explor Immunol. 2025; 5: 1003202.
- Rogovik AL, Carleton B, Solimano A, Goldman RD. Palivizumab for the prevention of respiratory syncytial virus infection. Can Fam Physician. 2010; 56(8): 769-772.
- Otsubo R, Yasui T. Monoclonal antibody therapeutics for infectious diseases: Beyond normal human immunoglobulin. Pharmacol Ther. 2022; 240: 108233.
- Grace PS, Gunn BM, Lu LL. Engineering the supernatural: monoclonal antibodies for challenging infectious diseases. Curr Opin Biotechnol. 2022; 78: 102818.
- Pantaleo G, Correia B, Fenwick C, Joo VS, Perez L. Antibodies to combat viral infections: development strategies and progress. Nat Rev Drug Discov. 2022; 21(9): 676-696.
- Atanasov PY, Moneva-Sakelarieva MG, Kobakova YA, Ivanova SA, Becheva MV, Kirkova-Bogdanova AG, Chaneva MS, Atanasova VP, Todorov RF, Bashev NZ, Bashov IE, Ibrahim AH. Monoclonal antibodies: their place in applied medicine and their role in the newest infectious disease – history, present, and future. Literature review. Pharmacia. 2025; 72: 1-18.
- Pelfrene E, Mura M, Cavaleiro Sanches A, Cavaleri M. Monoclonal antibodies as anti-infective products: a promising future? Clin Microbiol Infect. 2019; 25(1): 60-64.
- Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM; BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med. 2021; 385(15): 1382-1392.
- Dluzynski D, Ssentongo P, Hale CM, Shaheen SK, Maglakelidze N, Sivik JM, Henao MP, Chinchilli VM, Paules CI. Dose-dependent impact of tixagevimab-cilgavimab as primary prevention against SARS-CoV-2 in immunocompromised individuals. Sci Rep. 2025; 15(1): 17578.
- Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, Kaiser F, Cohen R, Pinquier D, Felter CT, Vassilouthis NC, Jin J, Bangert M, Mari K, Nteene R, Wague S, Roberts M, Tissières P, Royal S, Faust SN; HARMONIE Study Group. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N Engl J Med. 2023; 389(26): 2425-2435.
- Giacobbe DR, Dettori S, Di Bella S, Vena A, Granata G, Luzzati R, Petrosillo N, Bassetti M. Bezlotoxumab for Preventing Recurrent Clostridioides difficile Infection: A Narrative Review from Pathophysiology to Clinical Studies. Infect Dis Ther. 2020; 9(3): 481-494.
- Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009; 361(2): 135-144.
- Yamamoto BJ, Shadiack AM, Carpenter S, Sanford D, Henning LN, O’Connor E, Gonzales N, Mondick J, French J, Stark GV, Fisher AC, Casey LS, Serbina NV. Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity. Antimicrob Agents Chemother. 2016; 60(10): 5787-5795.
- Saxena D, Kaul G, Dasgupta A, Chopra S. Atoltivimab/maftivimab/odesivimab (Inmazeb) combination to treat infection caused by Zaire ebolavirus. Drugs Today (Barc). 2021; 57(8): 483-490.
- Grinsztejn B, Appay V, Bekker LG, Beyrer C, Donnell D, Sanchez J, Canagasabey D, Coutinho C, Ganor Y, Muturi-Kioi V, Ortblad KF, Cooney E, Devisich G, Ellenberg P, Ghiglione Y, K’Orimba K, Ssemambo PK, Ludwig-Barron NT, Mielke DK, Mullick R, Muthui MK, Radusky PD, Sendaula E, Tirmizi SRH, Sanchez AVM, Vega J, Pebody R. The science at HIVR4P 2024: The era of choice in biomedical HIV prevention. J Int AIDS Soc. 2025; 28(7): e70001.
- Skinner J, Kayentao K, Ongoiba A, Healy SA, Hu Z, Preston AC, Niangaly A, Schwabl P, Cisse H, Doumbo S, Doumtabe D, Traore A, Li S, Peterson ME, Seilie AM, Chavtur C, Staubus W, Chang M, Kelley K, Traore H, Djiguiba A, Keita M, Ouattara A, Doucoure M, Keita M, Diarra D, Sylla M, Diakite D, Konate M, Traore S, Zéguimé A, Dolo A, Neafsey DE, Murphy SC, Traore B, Seder RA, Crompton PD. Anti-sporozoite monoclonal antibody for malaria prevention: secondary efficacy outcome of a phase 2 randomized trial. Nat Med. 2025; 31(8): 2682-2690.
- Ramamurthy D, Nundalall T, Cingo S, Mungra N, Karaan M, Naran K, Barth S. Recent advances in immunotherapies against infectious diseases. Immunother Adv. 2020; 1(1): ltaa007.
- Patel PN, Dickey TH, Hopp CS, Diouf A, Tang WK, Long CA, Miura K, Crompton PD, Tolia NH. Neutralizing and interfering human antibodies define the structural and mechanistic basis for antigenic diversion. Nat Commun. 2022; 13(1): 5888.
- de Jong HK, Grobusch MP. Monoclonal antibody applications in travel medicine. Trop Dis Travel Med Vaccines. 2024; 10(1): 2.
- Cruz-Teran C, Tiruthani K, McSweeney M, Ma A, Pickles R, Lai SK. Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy. Adv Drug Deliv Rev. 2021; 169: 100-117.
- Berry JD, Gaudet RG. Antibodies in infectious diseases: polyclonals, monoclonals and niche biotechnology. N Biotechnol. 2011; 28(5): 489-501.


