Gaps in Biopharma Drug Development Strategy
- Biopharma Drug Development Industry report reveals that 25% of drug-target pairs in biopharma R&D are concentrated on just 38 biological targets.
- Only 350 novel targets have emerged in the past five years, with most still in preclinical stages. And only 30 new targets entered the pipeline in 2024.
- Early partnerships with CDMO from preclinical stage can accelerate development, de-risk emerging mechanisms, and improves success rates for novel modalities. While new modalities like bispecifics and ADCs are growing, they often recycle familiar targets.
The Crowding Effect in Biopharma Drug Development
A new analysis by L.E.K. Consulting underscores a growing imbalance in drug development pipelines: one-quarter of the ~13,600 drug-target pairs currently in preclinical and clinical development are linked to just 38 biological targets. Despite the massive expansion of the overall R&D landscape, the concentration of innovation around a few well-worn pathways signals a troubling bottleneck in scientific diversification.
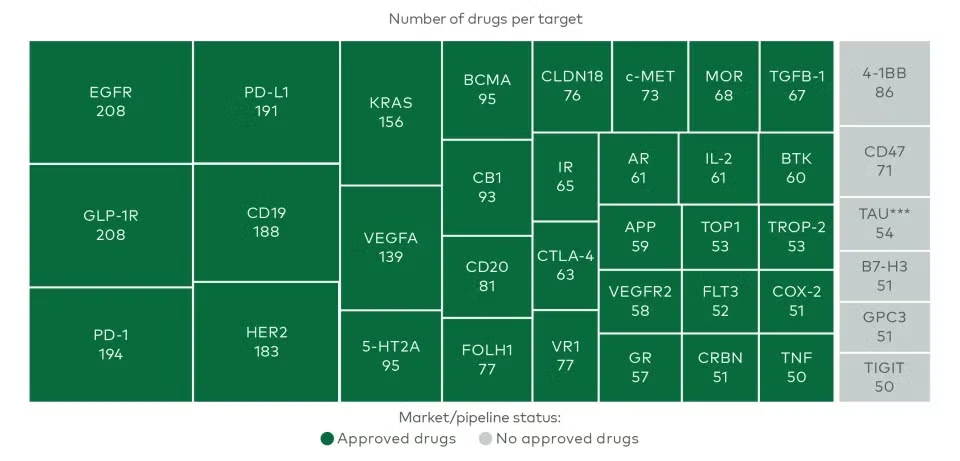
Figure 1. Worldwide preclinical and clinical R&D pipeline targets with 50+ associated drugs.
Despite a threefold increase in early-stage venture capital over the past decade, the number of novel biological targets entering the pipeline each year has plummeted from around 100 a year pre-COVID to only 30 in 2024. Report clearly indicates that innovation in what biopharma is targeting has failed to keep pace with how those targets are modulated or delivered.
This gap is not due to an absence of capital. Series A investments in life sciences have continued to grow steadily (by about 18% annually). But rather than supporting a broad base of novel mechanisms, this capital is increasingly concentrated in fewer startups, many of which pursue known targets with proven commercial pathways.
The Hidden Risks Behind Biologics Development Overlap
More than 10,000 active drug programs focus on targets with known mechanisms, while novel targets are increasingly rare. L.E.K. Consulting identified only about 350 novel targets introduced between 2020 and 2024, primarily in oncology, immunology, metabolism, and neuroscience. These areas dominate investment despite other therapeutic categories also suffering from unmet clinical needs. Typically, targets with less characterized biology require more effort and investment, so economically it is not worthwhile to engage in their development.
Of those 350 novel targets, nearly 70% remain in preclinical stages, with only a fraction advancing into Phase 1 trials. Promising candidates such as ALKBH5, CD84 and NEK7 are still early in their clinical journey, and it remains uncertain how many will reach late-stage development. The attrition rate for novel biology remains high further reinforcing investor conservatism.


Herd mentality in biotech isn’t just inefficient. It’s a very dangerous game to get involved in.


This dynamic creates a self-fulfilling prophecy:
- Novel targets struggle for funding due to risk
- The limited number that do get funded face scientific headwinds
- Overall low success rate further disincentivizes investment
The result is a pipeline that grows in size but narrows in biological scope.
Future of Biopharma Drug Development
Biopharma pipelines are expanding in size but shrinking in mechanistic diversity, with development efforts focused on a narrow set of validated targets. The industry is at a strategic crossroads, where scientific opportunity is outpacing operational readiness. Emerging therapeutic modalities, from antibody-drug conjugates (ADCs) to targeted protein degraders (PROTACs), demand entirely new drug development model. With the average cost of bringing a drug to market exceeding $2.5 billion and timelines still spanning a decade, the pressure to innovate more efficiently has never been greater.
Current R&D pipelines are heavily concentrated around a few well-known biological targets, contributing to redundancy and commercial saturation. In this environment, differentiation is king – not just in molecular design, but in development strategy. Investors and boards are increasingly scrutinizing not only therapeutic innovation but also the operational execution of development programs. Scalability, manufacturability, and regulatory readiness are now key to commercial success, even in early-phase ventures.
Rapidly establishing partnerships with CDMOs from the early stages of biologic drug development (preclinical studies, Phase 1 clinical trials) can be a significant competitive advantage. Biotech companies gain access to advanced analytical gene-to-vial platforms, and scalable biomanufacturing technologies. This approach mitigates technical risk, accelerates timelines, and ensures a seamless transition from clinical to commercial scale. Process development acceleration provide access to platform technologies and analytical expertise essential for novel modalities like bispecifics, or ADCs.
More than half of the estimated 4,500 druggable proteins in the human genome remain untouched by drug development. Unlocking these could be the key to treating diseases that today have no effective therapies. To do so, biopharma must pair advanced technologies with smarter early-stage capital allocation and stronger academic-industry partnerships. Only by collectively lowering the risk barrier can novel targets receive the investment and validation they need to succeed.
Prepared by:

Jakub Knurek
Marketing Specialist
Sources and further reading
- Mancuso M, Jacquet P, Brau R, Dhulesia A, Srinivasan A. Is Biopharma Doing Enough to Advance Novel Targets? L.E.K. Insights. L.E.K. Consulting. 2025; 27(34).
- Ranbhor R. Advancing Monoclonal Antibody Manufacturing: Process Optimization, Cost Reduction Strategies, and Emerging Technologies. Biologics. 2025; 19: 177-187.
- Tuszyner A. Innovative biologics – expected drug approvals in 2025. Mabion Science Hub. 2025.
- Kwak JW, Houghton AM. Targeting neutrophils for cancer therapy. Nat Rev Drug Discov. 2025.
- Chen L, Bush B, Brochu M, King G. New Drug Modalities 2024. Boston Consulting Group. 2024.
- Loscalzo J. Molecular interaction networks and drug development: Novel approach to drug target identification and drug repositioning. FASEB J. 2023; 37(1): e22660.