EMA and FDA investigating cancer signal for CAR-T therapies
- EMA and FDA have started a safety review of CAR-T therapies following the notification of 23 cases of T-cell malignancies. The investigation involves all registered CAR-T products, which are used for treating relapsed and treatment-resistant hematologic malignancies.
- If carcinogenicity of CAR-T therapy is confirmed, EMA and FDA would introduce appropriate regulatory actions, including modification of product characteristics and possibly restriction of use to the selected patient groups. Regardless of the review outcome, patients must be now closely monitored for the potential cases of secondary leukemia or lymphoma.
- Although the causal relationship between CAR-T and T-cell malignancies is still uncertain, the announcement of safety review dealt a major blow to CAR-T researchers and pharma companies, who following the remarkable results in treating blood cancers, count on similar success in solid tumors.
First the FDA and now EMA. Investigations on the possible link between CAR-T therapies and secondary T-cell malignancies were started by both agencies, after 23 cases were reported from clinical trials and via passive surveillance. The inquiries involve all CAR-T products approved in the European Union and United States:
- Abecma (Bristol Myers Squibb)
- Breyanzi (Bristol Myers Squibb)
- Carvykti (Jannsen (J&J) and Legend Biotech)
- Kymriah (Novartis)
- Tecartus (Gilead Sciences Inc.)
- Yescarta (Gilead Sciences Inc.)
All above products consist of autologous T cells modified to target the BCMA and CD19 antigens expressed on malignant cells. They are indicated for the treatment of various hematologic malignancies: B-cell lymphoma, B-cell leukemia, follicular lymphoma, multiple myeloma and mantle cell lymphoma.
The EMA and FDA revealed little information on the reviewed cases and left no clue as to when this process could be finalized. Should a causal relationship between CAR-T and T-cell leukemias be confirmed, agencies may need to impose additional regulations, e.g. incorporate special warnings and requirement for lifetime monitoring of treated patients in the product characteristics, or restricting use to selected group of patients. Withdrawal of any of the registered products is highly improbable, if not impossible, given that for many patients CAR-T remains the last resort to win with cancer. In fact, the FDA stressed in their announcement, that the benefits of this therapy continue to outweigh the possible risks. After all, the potential risk of T-cell malignancy is low and distant in time, while the immediate risk of dying without taking CAR-T is disproportionately high for most treated patients.
Additional research will be needed to elucidate the relationship between CAR-T therapy and T-cell malignancies. Fortunately, papers shedding light on this issue are already showing up. In a recent report published in Nature Medicine, researchers describe a case of T-cell lymphoma occurring three months after the administration of CAR-T therapy for non-Hodgkin B-cell lymphoma. The close temporal relationship suggested that the received immunotherapy could be a culprit. However, initial suspicions were not confirmed in subsequent investigations, which revealed minimal expression of CAR transgene in lymphoma cells and presence of the same cell clone before CAR-T infusion, indicating that the therapy did not contribute to the development of new cancer. The researchers also quantified the overall risk of new malignancies after commercial CAR-T by inspecting the records of all patients treated at the University of Pennsylvania. After a median of 10.3 months of follow-up, 3.6% patients developed secondary cancers, including one case of T-cell lymphoma. Even if causation is confirmed, the absolute risk of lymphoma following CAR-T appears miniscule. Considering the previous use of cytotoxic chemotherapy, the 23 cases, which triggered safety review may turn out to be nothing more than a chance occurrence. Molecular analyses of reported lymphomas will play a pivotal role in resolving this issue.
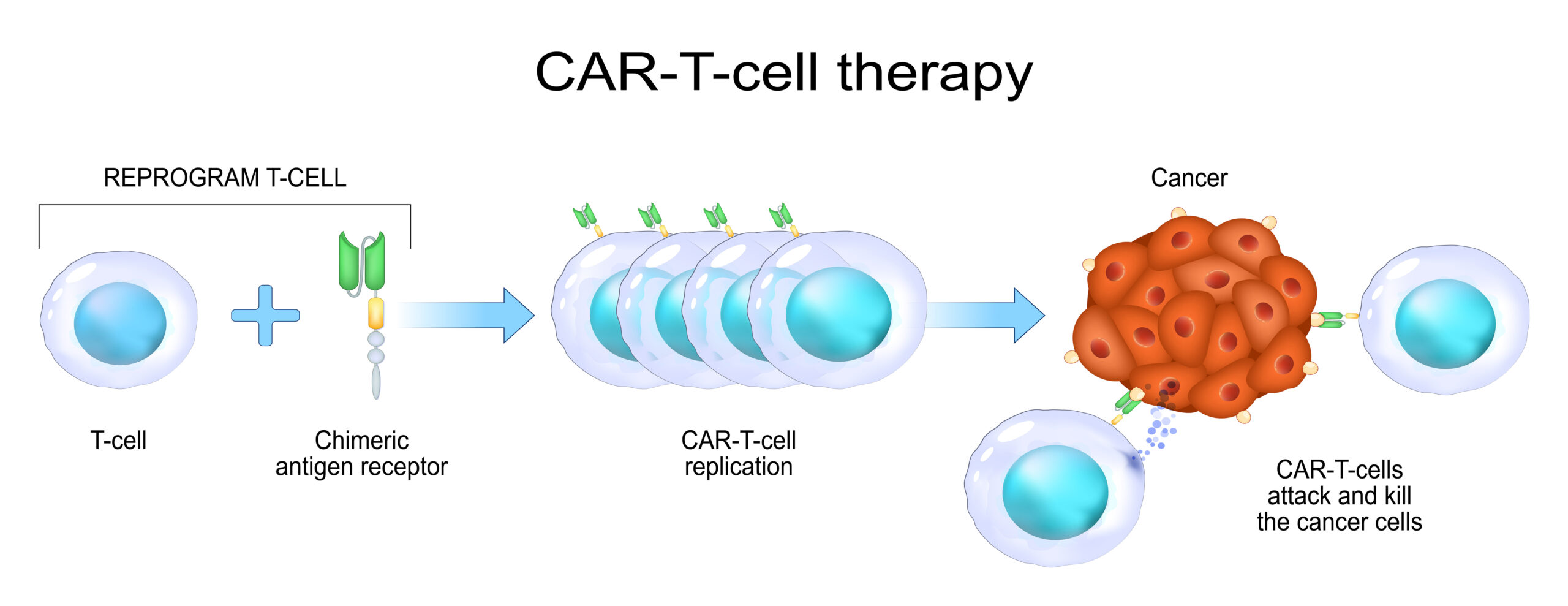
CAR-T is a breakthrough technology offering hope to patients with progressing or relapsing cancer, who exhausted all other therapeutic options. In CAR-T therapy, patient’s own T cells are collected and genetically modified under in vitro conditions to express the receptors targeting cancer antigens. After re-infusion, they act like soldiers whose only mission is to find and destroy malignant cells. This approach proved to be extremely effective, even in cancers resistant to conventional therapies. For example, Breyanzi (lisocabtagene maraleucel) was found to improve the event-free survival of patients with relapsed or treatment-resistant large B-cell lymphoma from 2.3 to 10.1 months.
The safety review by EMA and FDA came at a critical time for CAR-T therapies. Their use in hematology is expanding and many trials are underway to test CAR-T therapies in solid tumors. The researchers expect them to bring similar results as in hematological cancers, serving as a life belt to patients who went through all other treatment options. Although the CAR-T field received a major blow, outlook for the future remains positive and the therapy still holds much promise in the treatment of various cancers.
Prepared by:
References
- Food and Drug Administration (FDA). “FDA Investigating Serious Risk of T-cell Malignancy Following BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies (November 28, 2023).
- European Medicines Agency (EMA). “Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8-11 January 2024” (January 12, 2024).
- Jules A. “FDA CAR-T cell therapy warning: Are T cells malignant?” (December 4, 2023) Labiotech.eu.
- Ghilardi, Guido, et al. “T-cell Lymphoma and Secondary Primary Malignancy Risk After Commercial CAR T-cell Therapy.” Nature Medicine (2024): 1-1.
- Kamdar, Manali, et al. “Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial.” The Lancet 399.10343 (2022): 2294-2308.