First denosumab biosimilar approved by FDA
- Sandoz has just won the race for the first FDA-approved biosimilar to denosumab. The company successfully registered two biosimilars, Wyost and Jubbonti, each corresponding to a different formulation of the originator.
- Denosumab biosimilars received approval for all indications of the reference product and were granted with interchangeability status, which means that they can be substituted for the original denosumab without the permission from healthcare provider.
- Many other denosumab biosimilars are heading for approval in both the US and EU, but Sandoz, being first to cross the finish line, will likely seize the largest market share.
The race for the first biosimilar to denosumab, a widely prescribed medication for bone diseases, is now over. On March 5th, FDA granted licensure to two Sandoz products, Wyost and Jubbonti, which correspond to two distinct formulations of the original denosumab – Xgeva® and Prolia®. The former, a high-dose preparation (120 mg/1.7 ml), is used primarily in patients with bone metastases from solid tumors, while the latter, containing lower dose (60 mg in 1 ml), is indicated for postmenopausal osteoporosis and bone loss associated with hormonal therapy. Sandoz products received approval for all these indications. Moreover, the company has also managed to obtain interchangeability status, which means that pharmacies can substitute biosimilars for the reference product without the need for additional permission from prescribing physician.
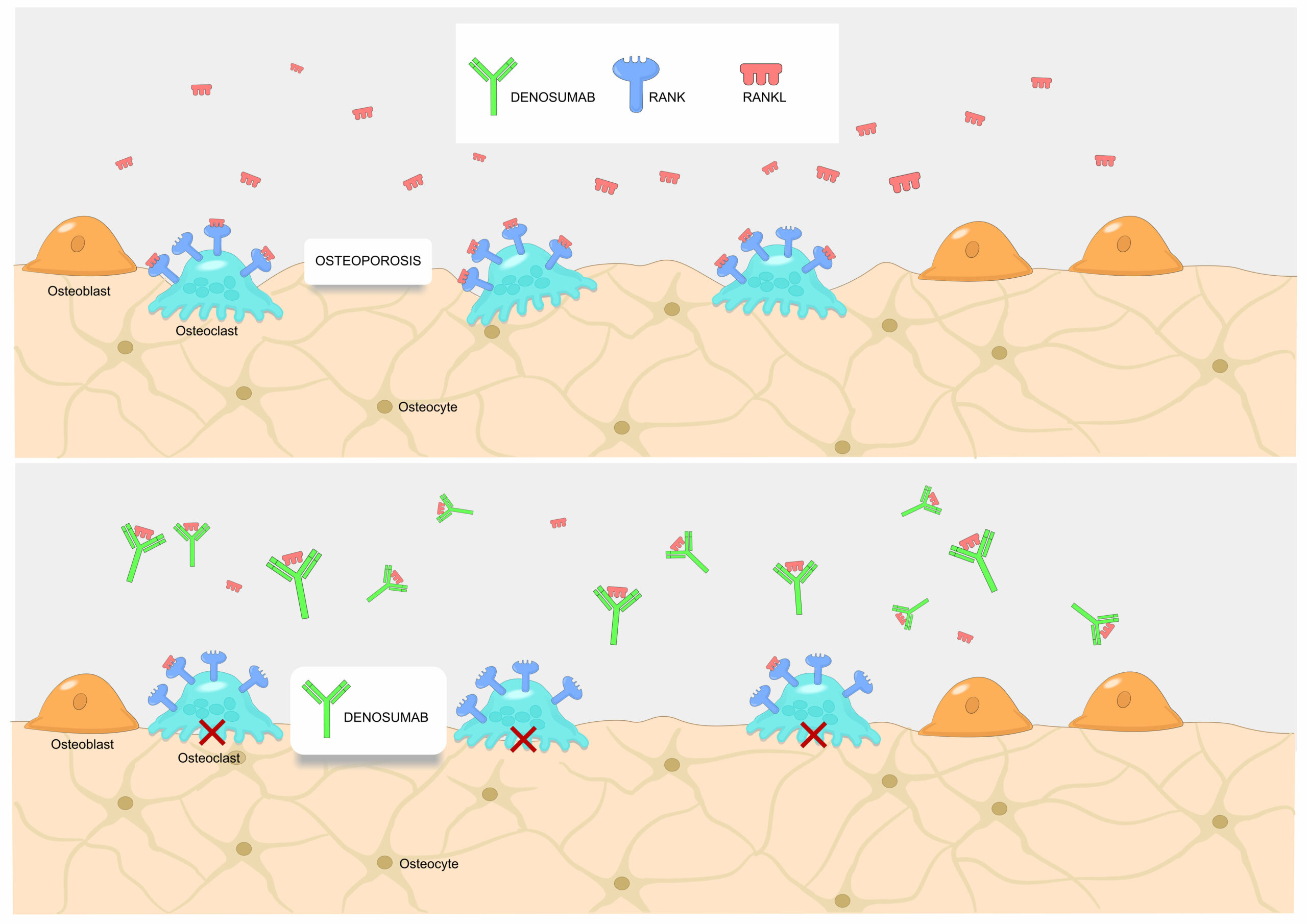
Denosumab is an extremely popular biologic used by millions of patients worldwide who suffer from excessive bone loss or cancer metastases to bones. It is a human monoclonal antibody targeting a protein called RANKL, which physiological role is to stimulate the formation and activity of osteoclasts – the cells responsible for bone resorption. Inhibition of RANKL by denosumab arrests this process and helps bones regain their mass. The clinical efficacy of denosumab, as observed in pre-licensure studies, is indeed remarkable and substantially higher than for traditional osteoporosis drugs.
International sales of Prolia® and Xgeva® reached record-breaking highs last year, soaring to $ 4 bln and $ 2.1 bln, respectively. The monumental commercial success of denosumab attracted numerous biosimilar companies to develop their own counterparts. Close to filing and obtaining FDA approval are biosimilar candidates from Celltrion (CT-P41), Gedeon Richter (RGB-14-P) and Samsung Bioepis (SB16) – all having completed pivotal Phase III studies, demonstrating therapeutic similarity to the originator. Therefore, Sandoz must brace for a strong competition in the near future from these well-established biosimilar companies. Nonetheless, being first on the finish line confers a high competitive advantage and opportunity to claim the largest market share, which is then difficult to regain by subsequent entrants. Experience from other biosimilars, such as rituximab, shows that the first companies reaching the market often dominate the biosimilar landscape for years, as they have time to establish brand recognition and gain trust among healthcare providers.
Fortunately, strong competition pays off for the patients. The biosimilars’ prices are currently about 30% lower than for the corresponding reference products, and their predicted expansion over the next years is likely to push the prices even lower.
Prepared by:
Sources and further reading
- Sandoz media release “Sandoz receives FDA approval for first and only denosumab biosimilars.” March 5, 2024. Link: https://www.sandoz.com/sandoz-receives-fda-approval-first-and-only-denosumab-biosimilars/.
- Amgen press release “Amgen reports fourth quarter and full year 2023 financial results” Link: https://www.amgen.com/newsroom/press-releases/2024/02/amgen-reports-fourth-quarter-and-full-year-2023-financial-results.
- Vogg, Barbara, et al. “Pharmacokinetics and pharmacodynamics of the proposed biosimilar denosumab GP2411 and reference denosumab in healthy males.” Expert Opinion on Biological Therapy just-accepted (2024).
- Cummings, Steven R., et al. “Denosumab for prevention of fractures in postmenopausal women with osteoporosis.” New England Journal of Medicine 361.8 (2009): 756-765.
- Kim, Anhye, et al. “A randomized, double-blind, single-dose, phase 1 study comparing the pharmacokinetics, pharmacodynamics, safety, and immunogenicity of denosumab biosimilar CT‑P41 and reference denosumab in healthy males.” Expert Opinion on Biological Therapy just-accepted (2024Drugs 37.6 (2023): 855-871.
- ClinicalTrials.gov NCT05087030 “Comparative Efficacy and Safety Study of RGB-14-P and Prolia® in Women With Postmenopausal Osteoporosis.” Link: https://clinicaltrials.gov/study/NCT05087030.
- Lee, Hyun A., et al. “A phase I, randomized, Double-blind, single-dose pharmacokinetic study to evaluate the biosimilarity of SB16 (proposed denosumab biosimilar) with reference denosumab in healthy male subjects.” Expert Opinion on Investigational Drugs 32.10 (2023): 959-966.
- Hillhouse, Erin, et al. “The economic impact of originator-to-biosimilar non-medical switching in the real-world setting: a systematic literature review.” Advances in Therapy (2022): 1-33.