Mammalian cell lines as a platform for protein vaccine production
Cell culture, Drug substance, Manufacturing, Proteins, Vaccines
- Recombinant protein vaccines are mostly produced using bacterial or yeast platforms, but mammalian cell lines such as Chinese Hamster Ovary (CHO) are recently gaining traction owing to their high productivity and ability to perform complex post-transcriptional modifications.
- As of May 2025, there are only several protein vaccines derived from mammalian cell lines approved by the EMA and/or FDA. These include vaccines against herpes zoster (Shingrix), respiratory syncytial virus (Abrysvo, Arexvy), hepatitis B (Prehevbrio) and COVID-19 (Bimervax), all of which were introduced in the last eight years.
- Numerous CHO-produced vaccines are currently in development, including breakthrough products against HIV-1 and cytomegalovirus infections. Their approval may set stage for even a broader adoption of mammalian cells in vaccine manufacturing.
From Jenner to recombinant proteins
Ever since the groundbreaking invention of smallpox immunization by Edward Jenner in the late 18th century, vaccines have remained a cornerstone of infectious disease prevention. Despite occasional opposition from a small, but vocal group of “skeptics”, their impact on reducing the morbidity and mortality from childhood infections has been unequivocal (Figure 1). Thanks to the widespread immunization campaigns across Europe, we no longer encounter diseases like smallpox or polio, which used to kill and produce lifelong disability in thousands of young children annually. While the danger of remaining infectious diseases such as diphtheria, tetanus, measles or rubella still persists, their public health impact has been dramatically reduced. Meanwhile, other pathogens, including cytomegalovirus and HIV, remain at the forefront of vaccine research.
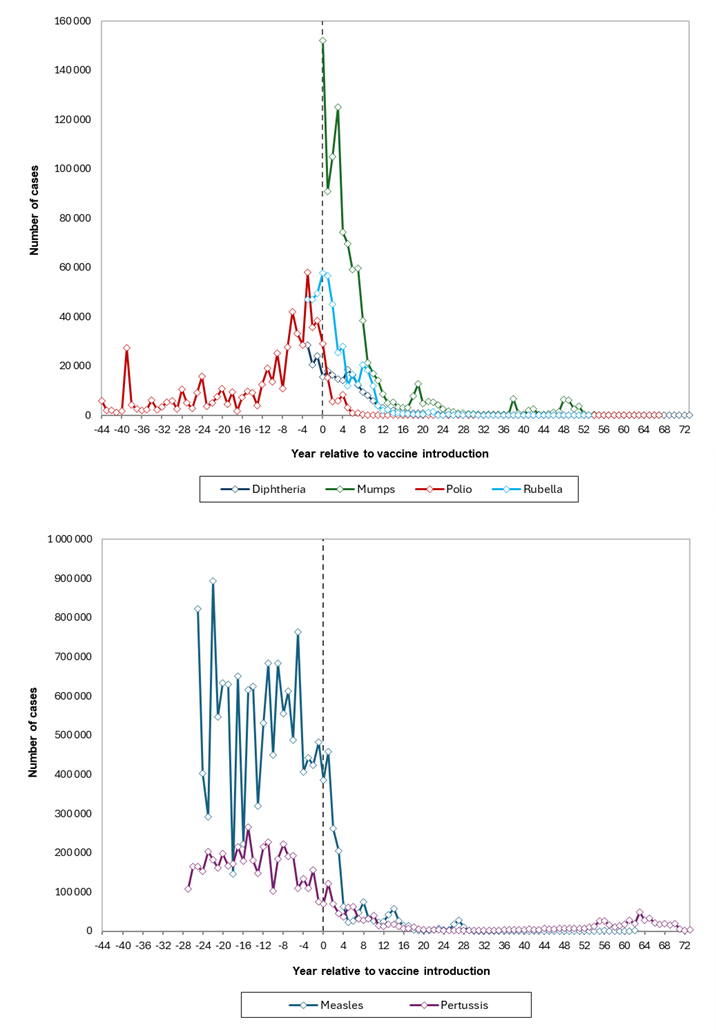
Decades of research have given rise to multiple vaccine platforms – from traditional inactivated or live-attenuated formulations to next-generation technologies, such as mRNA and viral vector-based vaccines, which were deployed on a massive scale during the COVID-19 pandemic.1 Each brings unique strengths, challenges and distinct manufacturing, quality and regulatory considerations.2
Among these platforms, recombinant protein vaccines stand out as a particularly compelling option. Produced with the use of well-established techniques and expression systems (mammalian included), they offer numerous advantages over other types and are still relevant to vaccine research, even in the age of mRNA and vector technologies.3
Enduring strengths of protein-based vaccines
Unlike inactivated whole-cell or live-attenuated vaccines, protein vaccines contain only well-characterized antigen(s) that are known to induce effective immune responses.4 Being devoid of unnecessary viral or bacterial components that may produce inflammation without affording extra protection, they usually have an excellent safety profile, with very low rates of severe systemic reactions.5 In this respect they also outperform mRNA vaccines, which tend to cause significant flu-like symptoms after second and subsequent doses.6
The safety and efficacy of protein vaccines are backed by nearly four decades of clinical use, spanning various pathogens and formulations.7 They have been consistently demonstrated to induce strong and long-lasting protection, especially when sufficiently immunogenic antigen is combined with a potent adjuvant.8 Traditional adjuvants such as aluminum salts (e.g., aluminum phosphate, Al(PO4)3), are most commonly used in the currently marketed products, although newer proprietary adjuvants, like GSK’s AS03 or Novavax’s Matrix-M, are increasingly encountered in novel formulations.9 Particularly robust and targeted immune activation, similar to the one generated during infection in a quantitative and qualitative sense, can be achieved by immunization with protein virus-like particles (VLPs).10 VLPs are self-assembling particles composed of viral proteins, which are structurally similar to viruses but owing to the lack of genetic material, are unable to reproduce.11 Such particles are recognized and processed by the human immune system in a manner similar to natural viruses, including internalization by dendritic cells and presentation to cytotoxic CD8+ T cells via MHC class I pathways.12 This mimcry enhances both cellular and humoral immune mechanisms, providing outstanding protection against viral infections.13
Beyond immunological advantages, recombinant protein vaccines offer also practical benefits. Their non-replicating nature makes them suitable for immunocompromised individuals, who cannot receive live attenuated vaccines such as Sabin’s poliomyelits vaccine, which can occasionally revert to virulent form and cause paralysis.14 As manufacturing does not involve live or infectious agents, recombinant protein technologies minimize biosafety risks inherent to inactivated or attenuated vaccine production.15 With the current control mechanisms and strict regulations, the risk of inadvertent exposure of staff and/or patients to fully competent pathogens (e.g., due to ineffective virus neutralization or attenuation) is extremely low, but used to be a major problem in the early days of vaccinology (see e.g., Cutter incident in 1955, where incomplete inactivation led to polio outbreaks).16
Long-term clinical experience and well-established manufacturing methods make protein vaccines a viable alternative for physicians and patients who hesitate to use newer mRNA or viral vector technologies.17 These features proved crucial during the COVID-19 pandemic when significant segment of population opted to be vaccinated with Nuvaxovid vaccine (manufactured by Novavax) containing recombinant spike protein rather than Comirnaty (Pfizer) or Spikevax (Moderna).18 This trust in protein-based platforms is a significant factor, which should be taken into consideration when planning epidemic responses.19
From a production standpoint, recombinant protein vaccines are cost-effective and scalable. Their manufacturing costs generally fall between traditional inactivated vaccines and more resource-intensive mRNA or viral vector products.20 Moreover, their intrinsic physical stability can simplify cold-chain logistics and reduce distribution costs, especially in low- and middle-income regions.21
The manufacturing flexibility of recombinant platforms allows rapid adaptation to emerging variants by simply substituting the antigenic sequence.22 Protein vaccines are also amenable to combination formulations, enabling multivalent or pan-pathogen approaches.23 However, the same advantage applies also to mRNA vaccines.24
Mapping the market
Recombinant protein technology is an important component of the global vaccine portfolio.3 As shown in Table 1, 17 of the 98 vaccines currently licensed by the U.S. Food and Drug Administration (FDA) contain recombinant protein antigens, making up a significant and growing share of approved immunizations.25 The list includes several widely used products that figure in standard pediatric and adult immunization schedules e.g., hepatitis B or HPV vaccines.26 Billions of doses of these products have been distributed worldwide, reflecting their pivotal role in preventive medicine.27 Most of the vaccines in Table 1 are also approved for use in the European Union.28
| Vaccine | Trade name (manufacturer) | Year of introduction | Antigen(s) | Adjuvant(s) | Expression system |
|---|---|---|---|---|---|
| Chikungunya Vaccine, Recombinant | Vimkunya® (Bavarian Nordic A/S) | 2025 | Purified virus-like particles (VLPs) containing 3 chikungunya virus (CHIKV) structural proteins: Capsid (C), Envelope 1 (E1), Envelope 2 (E2) | Aluminum hydroxide | HEK |
| Diphtheria & Tetanus Toxoids & Acellular Pertussis Vaccine Adsorbed, Hepatitis B and Inactivated Poliovirus Vaccine Combined | Pediarix® (GlaxoSmithKline) | 2001 | Diphtheria toxoid, tetanus toxoid, acellular pertussis antigens (PT, FHA and pertactin), recombinant HepB surface antigen, inactivated poliovirus | Aluminum hydroxide Aluminum phosphate | Yeast (S. cerevisiae) – HepB component only |
| Hepatitis A Inactivated and Hepatitis B (Recombinant) Vaccine | Twinrix® (GlaxoSmithKline) | 1997 | Inactivated HepA virus, recombinant HepB surface antigen | Aluminum hydroxide Aluminum phosphate | Yeast (S. cerevisiae) – HepB component only |
| Hepatitis B Vaccine (Recombinant) | Recombivax HB® (Merck) | 1986 | Recombinant HepB surface antigen (HbSAg) | Aluminum hydro-phosphate sulphate | Yeast (S. cerevisiae) |
| Hepatitis B Vaccine (Recombinant) | Prehevbrio® (VBI Vaccines) | 2021 | HepB surface antigens (S, pre-S2, pre-S1) | Aluminum hydroxide | Chinese Hamster Ovary (CHO) |
| Hepatitis B Vaccine (Recombinant) | Engerix-B® (GlaxoSmithKline) | 1989 | HepB surface antigen (HbSAg) | Aluminum hydroxide | Yeast (S. cerevisiae) |
| Hepatitis B Vaccine (Recombinant), Adjuvanted | Heplisav-B® (Dynavax) | 2017 | HepB surface antigen (HbSAg) | CpG 1018 | Yeast (Hansenula polymorpha) |
| Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant | Gardasil® (Merck) | 2006 | Virus-like particles (VLPs) of the L1 protein of HPV types 6,11,16,18 | Aluminum hydro-phosphate sulphate | Yeast (S. cerevisiae) |
| Human Papillomavirus 9-valent Vaccine, Recombinant | Gardasil 9® (Merck) | 2014 | Virus-like particles (VLPs) of the L1 protein of HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58 | Aluminum hydro-phosphate sulphate | Yeast (S. cerevisiae) |
| Human Papillomavirus Bivalent (Types 16, 18) Vaccine, Recombinant | Cervarix® (GlaxoSmithKline) | 2009 | HPV L1 protein of types 16 and 18 | AS04 | Rix4446 (Trichoplusia ni) |
| Influenza Vaccine (Trivalent) | Flublok® (Protein Sciences) | 2013 | Influenza virus hemagglutinin (HA) from two A and one B types | No adjuvant | ExpresSF+ (derived from Sf9) |
| Influenza Vaccine (Quadrivalent) | Flublok Quadrivalent® (Protein Sciences) | 2016 | Influenza virus hemagglutinin (HA) from two A and two B types | No adjuvant | ExpresSF+ (derived from Sf9) |
| Meningococcal Group B Vaccine | Bexsero® (GlaxoSmithKline) | 2015 | Neisserial adhesin A (NadA), Neisserial Heparin Binding Antigen (NHBA), Factor H binding protein (fHbp), Outer Membrane Vesicles (OMV) | Aluminum hydroxide | Escherichia coli |
| Meningococcal Group B Vaccine | Trumenba® (Pfizer) | 2014 | Lipidated factor H binding protein (fHbp) variants from N. meningitidis type B (A05 and B01) | Aluminum phosphate | Escherichia coli |
| Respiratory Syncytial Virus Vaccine | Abrysvo® (Pfizer) | 2023 | Stabilized prefusion F antigen of RSV type A and B | No adjuvant | Chinese Hamster Ovary (CHO) |
| Respiratory Syncytial Virus Vaccine, Adjuvanted | Arexvy® (GlaxoSmithKline) | 2023 | Stabilized prefusion F antigen of RSV type A (RSVPreF3) | AS01E | Chinese Hamster Ovary (CHO) |
| Zoster Vaccine Recombinant, Adjuvanted | Shingrix® (GlaxoSmithKline) | 2017 | Glycoprotein E (gE) from varicella-zoster virus (VZV) | AS01B | Chinese Hamster Ovary (CHO) |
In terms of expression platforms, antigens in these 17 FDA-approved recombinant vaccines are produced using a range of biological systems: 7 in yeast cells (e.g., Saccharomyces cerevisiae), 5 in mammalian cell lines, 3 in insect cell lines and 2 in bacteria.15,25,29 All but one of the mammalian cell-derived products use Chinese Hamster Ovary (CHO) expression system: Prehevbio (hepatitis B vaccine), Abrysvo (RSV vaccine), Arexvy (RSV vaccine) and Shingrix (herpes zoster vaccine).30 Notably, all vaccines manufactured in CHO cells received approval within the last eight years, with Shingrix leading the way in 2017.31
The broader vaccine market has seen accelerated growth over the past decade, driven mostly by the COVID-19 pandemic, which prompted unprecedented investment in vaccine research, development, and manufacturing infrastructure.32,33 Between 2012 and 2022, 31 new products were authorized in the EU, including seven against COVID-19.28,33 A significant portion of these approvals were subunit vaccines (64.5%), with 10 products (32.3%) utilizing recombinant DNA technology as their production backbone.7
Beyond the U.S. and EU, recombinant vaccines are increasingly featured in the global immunization initiatives, with the WHO prequalifying several such products for broad international use.34
The case for CHO cell system in modern vaccine production
A recent shift toward mammalian cells
Although CHO cells dominate the therapeutic protein production, accounting for over 70% of all marketed products36, until recently they played little role in vaccine development.3 As mentioned above, of the 17 recombinant vaccines currently approved in the United States, only four (23.5%) are produced using CHO cells.25 However, this picture is rapidly changing. Three out of four recombinant vaccines approved since 2020 contain antigens manufactured in CHO cells, with the remaining one expressed in HEK, another mammalian expression platform.30 These 3 CHO-produced vaccines are:
- Prehevbrio® (VBI vaccines) – hepatitis B vaccine37,
- Abrysvo (Pfizer) – RSV vaccine38,
- Arexvy (GSK) – RSV vaccine.39
Another vaccine manufactured in CHO cell line is Bimervax, which is a COVID-19 vaccine containing recombinant SARS-CoV-2 spike (S) protein receptor-binding domain (RBD) fusion heterodimer.40 However, this vaccine is approved only in the EU.41
The shift towards CHO cells reflects a growing appreciation for their ability to support the expression of complex, conformationally sensitive antigens, especially viral glycoproteins that require human-like glycosylation pattern for immunogenicity.42,43 As recombinant protein vaccines target increasingly challenging pathogens – such as HIV, RSV, and CMV – CHO is emerging as a go-to system for native-like antigen expression.44
Why CHO cells are gaining ground
CHO cells bring several advantages that apply to both protein-based drugs and vaccine antigens:
- High and scalable productivity: Typical yield of 0.1-1.0 g/L in batch and 1.0-10.0 g/L in fed-batch cultures. Easy scale-up to late clinical and commercial phases.45
- Human-like posttranslational modifications: Including disulfide bond formation, protein folding, proteolytic processing, glycosylation.43 While some differences do exist, they can be addressed by using engineered cell lines.46
- Reduced contamination risk: CHO cells are not permissive to most human viruses.47
- Long-term and stable expression: Driven by the availability of well-optimized vectors and promoters.48
- Multi-year experience in biopharmaceutical use.49
Another compelling, but often underappreciated, advantage of using CHO cells is their universal acceptance by regulatory bodies.50 Alongside other mammalian cell lines like HEK293, CHO cells are among the most widely adopted expression systems in the biopharmaceutical industry.51 Their molecular biology has been extensively characterized, reducing the risk of unforeseen issues during drug or vaccine development. This established trust simplifies the regulatory approval process, as agencies are familiar with these systems and their safety profiles.52 In contrast, employing innovative but less conventional hosts might sometimes improve process efficiency but can also increase the complexity of required characterization studies.53 This often leads to additional scrutiny from regulators, potentially delaying approval timelines due to questions about safety, consistency, or scalability.54
Alternative expression systems for vaccine production, including bacterial, yeast and insect cells, are still valued for their rapid growth, high yields and cost-effectiveness.15 However, each of them has significant drawbacks that often position CHO cells as a preferable option, despite their lower productivity and higher costs.7
Escherichia coli, a staple in protein production, lacks the post-translational mechanisms needed for optimal protein folding and glycosylation.55 Many viral antigens, such as the SARS-CoV-2 spike protein and the RSV fusion protein, are in fact glycoproteins that undergo glycosylation in human cells to trigger a robust immune response.56 Since E. coli is unable to glycosylate proteins, it is unsuitable for producing these antigens in their native form.57 Additionally, lack of protein secretion in E. coli may cause aggregation of overexpressed proteins and formation of inclusion bodies.58 Complex purification and refolding steps that increase both cost and process intricacy, may have to be added in order to maintain satisfactory yields.59
Although yeast and baculovirus-transfected insect cells (e.g., Sf9, Sf21) can perform glycosylation, the resulting glycan structures differ markedly from those in mammalian cells.60 Insect cells typically produce simple paucimannose N-glycans, whereas mammalian cells, including CHO, generate more complex glycans including those with terminal sialic acids.61 These structural differences may reduce the immunogenicity and efficacy of vaccine antigens.62 Insect cell systems also face practical challenges, such as the need for costly culture media, baculovirus stock preparation, and relatively lower yields of secreted proteins, limiting their scalability.63
Human-like glycosylation of CHO cell lines is a valuable feature, particularly for producing structurally sensitive vaccines that require complex post-translational modifications.43,64 This capability is critical for antigens that must closely resemble their natural counterparts in human cells to elicit an effective immune response.65 One example of such antigen is BG505.SOSIP gp140 protein, a clinical-stage HIV vaccine candidate, which relies on extensive N-glycosylation at 20-30 sites as well as precise disulfide bonding and trimerization.66 This strong “glycan shield” and native-like trimer structure mimic the HIV virus evasion tactics, enabling the synthesis of broadly neutralizing antibodies.67 CHO cell lines are also essential for the production of FDA-licensed RSV vaccines (Abrysvo and Arexvy), whose protective efficacy depends on correct folding and glycosylation of a trimeric F protein in the specific pre-fusion conformation.68
Aside from the relevant differences in post-translational processing, insect cell lines (Sf9, Sf21 and other) have additional limitations as substrates for vaccine production, including expensive culture media, need for baculovirus stock preparation and characterization, as well as unsatisfactory yields for secreted proteins.69 Nevertheless, they are already employed in the production of several commercially successful vaccines such as Cervarix, a bivalent HPV vaccine manufactured in Rix4446 cell line derived from Trichoplusia ni70, and Nuvaxovid, a COVID-19 vaccine produced in Sf9 cells.71
Safety considerations and process control
One important caveat when using mammalian cell lines is the potential risk of introducing host and viral contaminants, which could potentially exhibit biological activity after entering the human organism.72 Specific precautions, purification steps and robust controls are required to be implemented in order to address this challenge and protect patients’ health.50
The issue of viral contaminants is particularly troublesome for VLP-based vaccines. Mammalian cells, including CHO, can release exosomes and endogenous retrovirus-like particles (RVLPs) into the culture medium, which may co-purify with vaccine VLPs, complicating their separation.43,73 Though RVLPs were proven to be non-infectious, they continue to stir some concern among drug regulators who mandate their removal, targeting concentrations below one particle per million doses.53 Each cell line must be screened for endogenous and exogenous viruses, and viral clearance steps must be integrated into downstream processing. Viral inactivation methods should be carefully adjusted to ensure safety without sacrificing antigen immunogenicity.74 For instance, the manufacturing process for Prehevbrio, an FDA-approved hepatitis B vaccine with VLPs composed of S, pre-S2 and pre-S1 antigens, includes formaldehyde treatment as the final inactivation step.37 This step was shown to eliminate contaminants while preserving antigen’s ability to stimulate an immune response.75
Emerging CHO-produced vaccines for high-need infections
A large number of protein vaccines are currently in development, many of which utilize CHO cell line for antigen production.3 This advanced approach has paved the way for promising vaccine candidates against diseases for which no effective immunization currently exists.44 Two such candidates, targeting Cytomegalovirus (CMV) and Human Immunodeficiency Virus (HIV), are described below. These viruses represent significant public health challenges due to their complex nature and profound impact on affected populations.4
Cytomegalovirus (CMV) vaccine
The journey to develop a vaccine against cytomegalovirus has been fraught with obstacles.76 The stakes are high: a successful CMV vaccine could protect young women from congenital infections that lead to severe neurodevelopmental disabilities in newborns, mirroring the transformative impact of the rubella vaccine.77 Unfortunately, early efforts using live-attenuated virus preparations failed to generate a robust and lasting immune response capable of preventing infection.78 Some research groups then turned to recombinant protein technology, which ultimately showed signs of efficacy in clinical trials.79
Utilizing a rational strategy to vaccine design, Chiron (later acquired by GlaxoSmithKline) developed a subunit vaccine containing a truncated version of CMV glycoprotein B (gB) produced in CHO cells.80 The gB vaccine has been combined and evaluated clinically with different adjuvants, such as aluminum, MF59 (oil-in-water emulsion) and most recently, AS01.81 In a phase II trial, MF-59 containing version was shown to be 50% effective in protecting adolescent women against CMV infection, albeit the protection was short-lived.79 Currently, GSK concentrates its efforts on AS01-adjuvanted vaccine composed of recombinant gB protein and CMV pentameric complex (gH/gL/pUL128-131).82 This complex, which can be efficiently produced in CHO cells, has been previously determined to be a major target for neutralizing antibodies in natural immunity and similarly to gB is an attractive vaccine target.83 A Phase I dose-ranging study of this preparation involving an unusually large cohort of 339 subjects has been recently completed, but results are not yet available (NCT05089630).84
In addition to subunit vaccines, researchers are exploring a virus-like particle version of the CMV vaccine, which expresses a modified gB protein.85 First-in-human studies have already been completed, demonstrating high immunogenicity and good tolerability of this VLP candidate.86 Upcoming studies will investigate whether this vaccine-induced immune response translates into effective protection against CMV infection.87
Human immunodeficiency virus (HIV) vaccine
Sadly, after nearly 40 years of painstaking efforts and billion-dolar investitions, we are still far away from licensing an effective HIV vaccine.88 Its development is one of the most significant and persistent scientific challenges in the history of vaccinology.89 In 2023 alone, there were around 1.3 million new HIV infections globally and despite highly effective retroviral therapy 630,000 people succumbed to its devastating consequences.90 The reasons behind this failure can be traced to inherent complexity of HIV. The virus is uniquely difficult to target due to its complicated lifecycle (e.g., genome integration and formation of latent reservoir of infected cells), high genetic diversity, effective immune evasion mechanisms and lack of natural sterilizing immunity.91 Despite little progress, scientists and pharmaceutical companies continue working on several vaccine candidates, one of which is the protein-based BG505 SOSIP.664 trimer vaccine, also produced in CHO cells.92 SOSIP stands for Soluble, Stabilized, Immunogenic Protein and refers to the genetically modified version of trimeric envelope protein (Env), capable of eliciting a broad spectrum of neutralizing antibodies.93 Complexity of BG505 SOSIP.664 antigen makes CHO a perfect cell substrate for its production.94 In the first-in-human trial, the proposed vaccine was found to be safe, well tolerated and immunogenic, supporting evaluation in further studies.95 A large Phase 3 efficacy trial, IMN004, is currently underway.96
Conclusions and future directions
Mammalian cells, particularly CHO, have been repeatedly validated as a robust platform for recombinant protein vaccine production.42 While they come with challenges such as increased costs and potential contamination risk, these drawbacks can be alleviated by clever process design and rigorous quality control measures.97 The substantial benefits they offer to manufacturers of complex viral vaccines including high yields, native-like structure and widespread regulatory acceptance, outweigh the relatively small limitations.49
Vaccines produced using CHO cells have already achieved remarkable success. Notable examples include vaccines against herpes zoster (Shingrix) and RSV (Abrysvo and Arexvy), which have already become blockbusters, being incorporated into national immunization programs across numerous countries.68 Their efficacy and safety has been confirmed in randomized clinical trials and real-world observational studies, which showed a substantial decline in the incidence of the targeted diseases after their introduction.98,99
These benefits translate into increasingly strong position of CHO cell line as a platform for vaccine production.36 Licensure of the vaccine candidates against the hitherto elusive pathogens, such as CMV or HIV may set stage for even broader option in both prophylactic and therapeutic pipelines.100
FAQ
Prepared by:

Adam Tuszyner
Regulatory Compliance Specialist
References
- Pollard, A. J., & Bijker, E. M. (2021). A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology, 21(2), 83-100.
- Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature, 586(7830), 516-527.
- Pollet, J., Chen, W. H., & Strych, U. (2021). Recombinant protein vaccines, a proven approach against pandemic and emerging pathogens. Trends in Biotechnology, 39(8), 756-772.
- Plotkin, S. A. (2014). Vaccines: past, present and future. Nature Medicine, 19(4), 423-427.
- U.S. Food and Drug Administration. (2021). Safety profile of recombinant protein vaccines: Hepatitis B and HPV vaccines. FDA Vaccine Safety Reports.
- Rubin, R. (2021). mRNA vaccines: Side effects and public perception. JAMA, 325(15), 1483-1485.
- Vetter, V., Denizer, G., Friedland, L. R., Krishnan, J., & Shapiro, M. (2018). Understanding modern-day vaccines: what you need to know. Annals of Medicine, 50(2), 110-120.
- Reed, S. G., Orr, M. T., & Fox, C. B. (2013). Key roles of adjuvants in modern vaccines. Nature Medicine, 19(12), 1597-1608.
- Keech, C., et al. (2020). Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine, 383(24), 2320-2332.
- Mohsen, M. O., Zha, L., Cabral-Miranda, G., & Bachmann, M. F. (2017). Major findings and recent advances in virus-like particle (VLP)-based vaccines. Seminars in Immunology, 34, 123-132.
- Roldão, A., et al. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149-1176.
- Bachmann, M. F., & Jennings, G. T. (2010). Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 10(11), 787-796.
- Zhao, Q., et al. (2013). Virus-like particle-based vaccines: challenges and opportunities. Human Vaccines & Immunotherapeutics, 9(11), 2383-2390.
- Centers for Disease Control and Prevention. (2020). Vaccine-derived poliovirus: Risks and management. CDC Global Health Reports.
- Nascimento, I. P., & Leite, L. C. (2012). Recombinant vaccines and the development of new vaccine strategies. Brazilian Journal of Medical and Biological Research, 45(12), 1102-1111.
- Offit, P. A. (2005). The Cutter incident: How America’s first polio vaccine led to the growing vaccine crisis. Yale University Press.
- Branswell, H. (2021). Why some people prefer protein-based COVID vaccines. STAT News.
- Heath, P. T., et al. (2021). Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. New England Journal of Medicine, 385(13), 1172-1183.
- Lazarus, J. V., et al. (2021). A global survey of potential acceptance of a COVID-19 vaccine. Nature Medicine, 27(2), 225-228.
- Gavi, The Vaccine Alliance. (2021). Vaccine manufacturing: challenges and solutions. Gavi Reports.
- World Health Organization. (2019). Vaccine cold chain logistics: Ensuring access in low-resource settings. WHO Technical Reports.
- Kanekiyo, M., et al. (2019). New vaccine design and delivery technologies. Journal of Infectious Diseases, 219(Suppl_1), S88-S96.
- Perrie, Y., et al. (2016). Vaccine adjuvant systems: enhancing the efficacy of subunit protein vaccines. International Journal of Pharmaceutics, 509(1-2), 1-9.
- Pardi, N., et al. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261-279.
- U.S. Food and Drug Administration. (2025). Vaccines licensed for use in the United States. FDA Vaccine Database.
- Centers for Disease Control and Prevention. (2023). Recommended child and adolescent immunization schedule. CDC Immunization Schedules.
- World Health Organization. (2022). Global vaccine action plan: Progress and challenges. WHO Immunization Reports.
- European Medicines Agency. (2024). Authorized medicines: Vaccines. EMA Medicines Database.
- Orenstein, W. A., Offit, P. A., Edwards, K. M., & Plotkin, S. A. (2024). Plotkin’s Vaccines, 8th edition. Elsevier Health Sciences.
- U.S. Food and Drug Administration. (2021-2023). Approval documents for Prehevbrio, Abrysvo, Arexvy, and Shingrix. FDA Biologics License Applications.
- Cunningham, A. L., et al. (2016). Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. New England Journal of Medicine, 375(11), 1019-1032.
- Branswell, H. (2021). How the COVID-19 pandemic transformed vaccine development. STAT News.
- European Medicines Agency. (2023). Annual report on new medicinal products authorized (2012–2022). EMA Annual Reports.
- World Health Organization. (2024). Prequalified vaccines list. WHO Prequalification Database. .
- Dey, Antu K., et al. “cGMP production and analysis of BG505 SOSIP. 664, an extensively glycosylated, trimeric HIV‐1 envelope glycoprotein vaccine candidate.” Biotechnology and bioengineering 115.4 (2018): 885-899.
- Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22(11), 1393–1398.
- VBI Vaccines. (2021). FDA approves Prehevbrio for hepatitis B. VBI Press Release.
- U.S. Food and Drug Administration. (2023). Abrysvo (Respiratory Syncytial Virus Vaccine) approval. FDA Approval Letter.
- U.S. Food and Drug Administration. (2023). Arexvy (Respiratory Syncytial Virus Vaccine) approval. FDA Approval Letter.
- European Medicines Agency. (2023). Bimervax: COVID-19 vaccine (recombinant, adjuvanted). EMA Product Information.
- European Medicines Agency. (2023). Bimervax authorization details. EMA Medicines Database.
- Zhu, J. (2012). Mammalian cell protein expression for biopharmaceutical production. Biotechnology Advances, 30(5), 1158–1170.
- Sanchez-Martinez, Zalma V., et al. “CHO cells for virus-like particle and subunit vaccine manufacturing.” Vaccine (2024).
- Walsh, G. (2018). Biopharmaceutical benchmarks 2018. Nature Biotechnology, 36(12), 1136–1145.
- Kim, J. Y., Kim, Y. G., & Lee, G. M. (2012). CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Applied Microbiology and Biotechnology, 93(3), 917–930.
- Hossler, P., Khattak, S. F., & Li, Z. J. (2009). Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology, 19(9), 936–949.
- Berting, A., Farcet, M. R., & Kreil, T. R. (2010). Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminants. Biotechnology and Bioengineering, 106(4), 598–607.
- Wurm, F. M., & Hacker, D. (2011). First CHO genome. Nature Biotechnology, 29(8), 718–720.
- Dumont, J., Euwart, D., Mei, B., Estes, S., & Kshirsagar, R. (2016). Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Critical Reviews in Biotechnology, 36(6), 1110–1122.
- U.S. Food and Drug Administration. (2018). Guidance for industry: Characterization and qualification of cell substrates for biologics. FDA Regulatory Guidance.
- Lai, T., Yang, Y., & Ng, S. K. (2013). Advances in mammalian cell line development technologies for recombinant protein production. Pharmaceuticals, 6(5), 579–603.
- Branswell, H. (2023). Why CHO cells are a regulatory favorite for biologics. STAT News.
- World Health Organization. (2013). Regulatory considerations for cell substrates used in vaccine production. WHO Technical Report Series.
- Marks, P. (2022). Challenges in approving novel cell substrates for vaccines. Medscape.
- Rosano, G. L., & Ceccarelli, E. A. (2014). Recombinant protein expression in Escherichia coli: Advances and challenges. Frontiers in Microbiology, 5, 172.
- Watanabe, Y., Bowden, T. A., Wilson, I. A., & Crispin, M. (2019). Exploitation of glycosylation in enveloped virus pathobiology. Biochimica et Biophysica Acta (BBA) – General Subjects, 1863(10), 1480–1497.
- Sahdev, S., Khattar, S. K., & Saini, K. S. (2008). Production of active eukaryotic proteins through bacterial expression systems: A review. Molecular and Cellular Biochemistry, 307(1–2), 249–264.
- Baneyx, F., & Mujacic, M. (2004). Recombinant protein folding and misfolding in Escherichia coli. Nature Biotechnology, 22(11), 1399–1408.
- Singh, S. M., & Panda, A. K. (2005). Solubilization and refolding of bacterial inclusion body proteins. Journal of Bioscience and Bioengineering, 99(4), 303–310.
- Jarvis, D. L. (2003). Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology, 310(1), 1–7.
- Moremen, K. W., Tiemeyer, M., & Nairn, A. V. (2012). Vertebrate protein glycosylation: Diversity, synthesis, and function. Nature Reviews Molecular Cell Biology, 13(7), 448–462.
- de Vries, R. P., et al. (2010). Glycan-dependent immunogenicity of recombinant viral glycoproteins. Journal of Biological Chemistry, 285(41), 31634–31642.
- Felberbaum, R. S. (2015). The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines. BioProcessing Journal, 14(2), 14–21.
- Varki, A. (2017). Biological roles of glycans. Glycobiology, 27(1), 3–49.
- Crispin, M., Bowden, T. A., Coles, C. H., & Harlos, K. (2018). Glycosylation in viral vaccine design. Nature Reviews Microbiology, 16(4), 238–247.
- Sanders, R. W., et al. (2013). A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing antibodies. PLoS Pathogens, 9(9), e1003618.
- Klasse, P. J., et al. (2018). The role of glycosylation in HIV-1 Env vaccine design. Trends in Microbiology, 26(7), 644–657.
- Papi, A., et al. (2023). Respiratory syncytial virus vaccines: A review of Arexvy and Abrysvo. Medscape.
- Cox, M. M. J. (2012). Recombinant protein vaccines produced in insect cells. Vaccine, 30(10), 1759–1766.
- European Medicines Agency. (2007). Cervarix: Human papillomavirus vaccine. EMA Product Information.
- Heath, P. T., et al. (2021). Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. New England Journal of Medicine, 385(13), 1172–1183.
- Berting, A., Farcet, M. R., & Kreil, T. R. (2010). Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminants. Biotechnology and Bioengineering, 106(4), 598–607.
- Roldão, A., et al. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149–1176.
- Gregersen, J. P. (2011). Vaccine production: Challenges in upscaling and ensuring safety. Vaccine, 29(17), 3171–3178.
- Aguilar, J. C., & Rodríguez, E. G. (2007). Vaccine adjuvants revisited: Hepatitis B vaccine. Vaccine, 25(30), 4983–4990.
- Schleiss, M. R. (2013). Cytomegalovirus vaccines: Progress and challenges. Expert Review of Vaccines, 12(12), 1449–1466.
- Centers for Disease Control and Prevention. (2022). Congenital CMV infection: Public health impact. CDC CMV Resources.
- Adler, S. P. (2013). Cytomegalovirus and pregnancy: Prospects for a vaccine. Clinical Infectious Diseases, 57(Suppl 4), S176–S181.
- Pass, R. F., et al. (2009). Vaccine prevention of maternal cytomegalovirus infection. New England Journal of Medicine, 360(12), 1191–1199.
- Griffiths, P. D. (2010). Cytomegalovirus vaccine development: A long road ahead. Journal of Infectious Diseases, 202(5), 667–669.
- Bernstein, D. I., et al. (2016). Safety and immunogenicity of a cytomegalovirus glycoprotein B vaccine with MF59 adjuvant. Vaccine, 34(3), 313–319.
- Gerna, G., & Lilleri, D. (2019). Human cytomegalovirus (HCMV) pentamer complex: A new target for vaccine development. Viruses, 11(8), 716.
- Chiuppesi, F., et al. (2017). Development of a synthetic pentamer vaccine against CMV. Vaccine, 35(32), 4109–4116.
- ClinicalTrials.gov. (2021). A study to evaluate a CMV vaccine in healthy adults (NCT05089630). NIH Clinical Trials Registry.
- Kirchmeier, M., et al. (2014). Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B. Vaccine, 32(25), 3039–3045.
- Branswell, H. (2022). CMV vaccine candidates show promise in early trials. STAT News.
- Schleiss, M. R. (2023). Advances in CMV vaccine development: What’s next? Medscape.
- Fauci, A. S., & Marston, H. D. (2014). Ending the HIV/AIDS pandemic: Follow the science. New England Journal of Medicine, 371(21), 1961–1963.
- Haynes, B. F., & Burton, D. R. (2017). Developing an HIV vaccine: Challenges and progress. Science, 355(6326), 1129–1130.
- World Health Organization. (2024). Global HIV & AIDS statistics — 2023 fact sheet. WHO HIV Reports.
- Klasse, P. J., et al. (2018). The role of glycosylation in HIV-1 Env vaccine design. Trends in Microbiology, 26(7), 644–657.
- Sanders, R. W., et al. (2013). A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing antibodies. PLoS Pathogens, 9(9), e1003618.
- Ward, A. B., & Wilson, I. A. (2017). The HIV-1 envelope glycoprotein structure: Nidus for neutralizing antibodies. Immunological Reviews, 275(1), 21–28.
- Julien, J. P., et al. (2013). Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science, 342(6165), 1477–1483.
- Bekker, L. G., et al. (2020). Safety and immunogenicity of a native-like trimeric envelope vaccine for HIV-1. Nature Medicine, 26(5), 681–687.
- ClinicalTrials.gov. (2023). IMN004: Phase 3 trial of BG505 SOSIP.664 HIV vaccine. NIH Clinical Trials Registry.
- European Medicines Agency. (2019). Quality control for biologics produced in mammalian cells. EMA Scientific Guidelines.
- Dumont, J., Euwart, D., Mei, B., Estes, S., & Kshirsagar, R. (2016). Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Critical Reviews in Biotechnology, 36(6), 1110–1122.
- Lal, H., et al. (2015). Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New England Journal of Medicine, 372(22), 2087–2096.
- Plotkin, S. A. (2023). The future of vaccines: Tackling CMV and HIV. Nature Reviews Immunology, 23(2), 67–68.
Related resources

The New Era of Biosimilar Development: Seizing the Opportunity Under EMA’s Streamlined Guidelines
Biosimilars, Clinical trials, Drug development, EMA, FDA, Mabion, Regulatory

The Role of Downstream Development in Ensuring Biologic Product Purity and Quality
Drug product, Manufacturing

Aseptic Filling in Modern Pharmaceutical Manufacturing
Drug product, Manufacturing