Quantitation of monoclonal antibodies in serum: new technologies ECL, Gyrolab, and LC-MS/MS vs gold standard ELISA
Bioanalytics
- Ligand binding assays and chromatographic methods (LC-MS/MS) remain the two most popular options for PK assessment in monoclonal antibodies pre-clinical and clinical studies.
- Different technology platforms claim various advantages over the gold standard ELISA, including increased sensitivity, decreased matrix interference, wider dynamic range, lower sample volume and easier implementation in a regulated environment.
- Fluorescence (Gyrolab) and electrochemiluminescent (MSD)-based detection methods have been implemented into traditional ELISA improving assay sensitivity, wider dynamic range and reduced background noise.
Monoclonal antibodies (mAbs) are a rapidly growing group of targeted therapeutics. In three decades, mAbs have revolutionized the treatment of different indications, including cancers, immune-mediated disorders, infectious diseases, cardiovascular/hemostasis, and others 1-3,10. In addition, new therapeutic modalities based on mAbs, including bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), have been approved or are in clinical trials 4,5. In 2021, the FDA announced that it approved the 100th monoclonal antibody therapeutic 6, and by 2023, about 170 mAb-based products are expected to be in the market (to date, are in regulatory review or are approved) 11. In 2022, 5 of the top 10 best-selling drugs in the global market were monoclonal antibodies 5.

The complexity and diversity of monoclonal antibodies requires the use of optimized bioanalytical assay platforms during their pre-clinical and clinical development. Regulatory requirements for non-clinical and clinical data, especially for pharmacokinetics (PK) assessment, are becoming increasingly restrictive. It is entirely understandable because during the development of therapeutic monoclonal antibodies, accurately determining the drug concentration is crucial in delineating the relationship between drug exposure and safety and efficacy 1-4. Consequently, significant efforts have also been spent on improving and standardizing the bioanalytical methodologies for quantifying monoclonal antibodies in different biological matrices.
In January 2021, the European Medicine Agency (EMA) replaced the EMA ‘Bioanalytical method validation – Scientific guideline’ with ICH guidance on validating biological assays ‘M10 Bioanalytical Method Validation and Study Sample Analysis’ 7,8. The US Food and Drug Administration (FDA) adopted the final ICH guidance in November 2022, which completed the international acceptance of a unified approach to validating and conducting bioanalytical assays. Among commonly used assays, Ligand Binding Assays and chromatographic methods remain the two most popular options for pharmacokinetics assessment.
Which assay type is the most appropriate for quantification of specific monoclonal antibodies?
The primary driver of bioanalytical strategy development is the intended use of the collected data. Depending on the development phase, the selected quantitative assay may capture either free or total protein. In certain studies, the availability of serum drug concentrations in free vs. bound states helps interpret PK, PD, and immunogenicity data and their interactions. Among the commonly used assays, Ligand Binding Assays (LBA) and chromatographic methods (LC-MS/MS) remain the two most popular options for PK assessment. Chromatographic techniques are best suited to measure the total drug concentrations because they detect the drug through signature peptides which presence is not affected by binding. Since LBA is based on the availability of binding sites on the analyte for both capture and detection reagents, it is commonly used to detect the free drug, but it could also be designed to measure the total drug. However, confirming the measured forms under the specified conditions can be technically challenging. The selected features of the assay that should be considered in the first stage of method selection for drug quantification are summarized in Table 1.
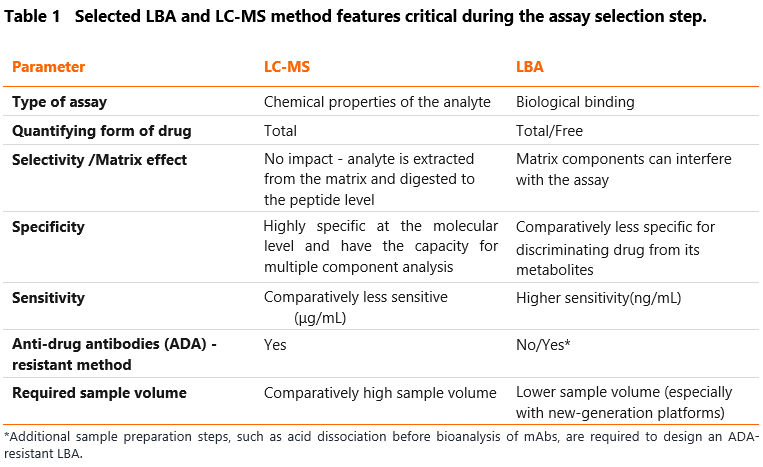
Anti-drug antibodies (ADA) could affect the accuracy of LBAs. In their presence, only the free form of the drug is measured since the therapeutic mAbs trapped by ADA are incapable of being quantified. Additional sample preparation steps, such as acid dissociation before bioanalysis of mAbs, may be required to design an ADA-resistant LBA assay. However, this could result in decreased sensitivity of the developed assay and a larger sample volume needed. Designing anti-drug antibodies (ADA)-resistant PK assay is critical in indications where the immunogenicity is predicted to be high. All stakeholders must appropriately communicate and understand the bioassay format (ADA-sensitive or ADA-resistant) as the program proceeds through drug development. As the chromatographic method includes pre-digestion of the sample, this method is considered ADA-resistant.
Switching from ELISA to advanced LBA platforms (Gyrolab and MSD)
ELISA is the gold standard approach for accurately quantifying large molecule-based therapeutics in the context of PK assessment for pre-clinical and clinical studies. As mentioned before, depending on the specific critical reagents used, ELISAs may detect one of the multiple forms of mAbs in circulation, including free mAb, antigen-bound mAb, total antibody, and ADA-mAb immunocomplex. Different technology platforms claim various advantages over the ELISA, including increased sensitivity, decreased matrix interference and wider dynamic range, lower sample volume and easier implementation in a regulated environment 13. These factors and others described herein are essential in selecting an appropriate platform for quantitative assay development and use.
Figure 1 shows the scheme of IgG quantitative assay with MSD (Meso Scale Discovery), Gyrolab xPlore, and LC-MS/MS platforms compared to gold standard ELISA. As presented, fluorescence (Gyrolab) and electrochemiluminescence (MSD)-based detection methods have been implemented into traditional ELISA, which results in improved assay sensitivity, wider dynamic range and reduced background noise.
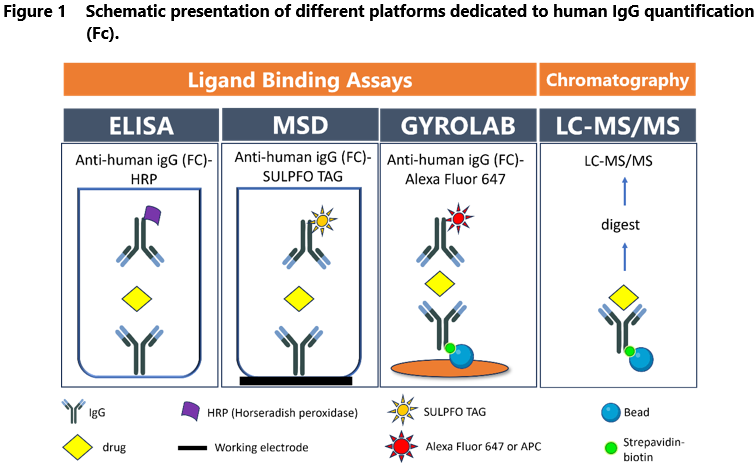
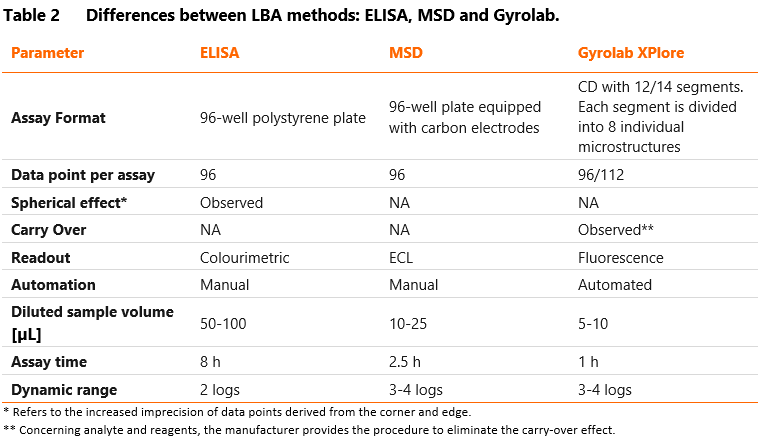
Gyrolab is a fully automated nanoliter-scale immunoassay that utilizes flow-through affinity columns to minimize the matrix effects and reduce assay background. It also provides excellent assay sensitivity and reproducibility without manual pipetting. The reaction is performed on a compact disc (Bioaffy CD), divided into 12-14 segments and 96-112 microstructures coated with streptavidin beads. The biotinylated capture antibody/target is immobilized on the beads to bind the analytes. The detection antibody is conjugated with fluorochrome. The CD is automatically transferred to a laser-induced fluorescence detector to determine the amount of fluorescence (i.e., the amount of captured analytes) per structure. Most importantly, microfluidic technology, automated control of centrifugal and capillary forces and a built-in fluorescence system minimize the required sample volumes (5-10 µL samples diluted to assay MRD).
Electrochemiluminescent technology with the SULFO-TAG label conjugated to detection antibodies (e.g., MSD) is another option to increase the LBA assay sensitivity. The reaction takes place on multi-well plates equipped with carbon electrodes. Carbon electrodes conduct electricity from the instrument to excite the SULFO-TAG, leading to light emission. Because MSD separates the light signal from electric stimulation, only conjugates near the electrode surface can be detected, resulting in low background noise. Thus, this method provides high sensitivity (pg/mL) and a broad dynamic range (3-4 logs). MSD platform has the significant disadvantage of its costs, which are much higher than ELISA plates.
Why did we transfer our ELISA method to Gyrolab platform?
Table 3 below presents the parameters obtained by Mabion for rituximab quantification assays (ELISA and the Gyrolab PK method). The desired sensitivity of the method was 1 µg/mL.
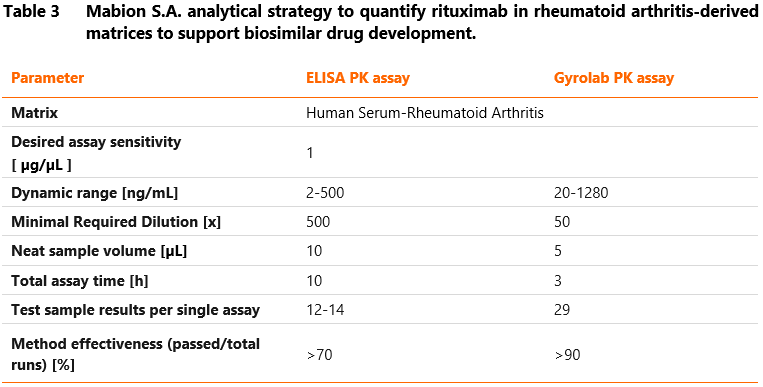
To conclude, from our experience, Gyrolab generates high-quality data in a short time, which makes it an ideal alternative to the manual ELISA method in support of GLP-compliant pre-clinical and clinical PK studies.
Prepared by:
Beata Talar
References
1. Zinn S, Vazquez-Lombardi R, Zimmermann C, Sapra P, Jermutus L, Christ D. Advances in antibody-based therapy in oncology. Nat Cancer. 2023 Feb;4(2):165-180. doi: 10.1038/s43018-023-00516-z.
2. Senolt L. Emerging therapies in rheumatoid arthritis: focus on monoclonal antibodies. F1000Res. 2019 Aug 30;8: F1000 Faculty Rev-1549. doi: 10.12688/f1000research.18688.1.
3. Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Infect Dis. 2022 Nov;22(11):e311-e326. doi: 10.1016/S1473-3099(22)00311-5. Epub 2022 Jul 5. Erratum in: Lancet Infect Dis. 2022 Sep;22(9):e239.
4. Kaplon H, Crescioli S, Chenoweth A, Visweswaraiah J, Reichert JM. Antibodies to watch in 2023. MAbs. 2023 Jan-Dec;15(1):2153410. doi: 10.1080/19420862.2022.2153410.
5. Brown A. Top product forecasts for 2023. Nat Rev Drug Discov. 2021 Dec;22(8). doi: 10.1038/d41573-022-00193-0.
6. Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. 2021 Jul;20(7):491-495. doi: 10.1038/d41573-021-00079-7.
7. EMA: Guideline on bioanalytical method validation. First published: 01/08/2011, Last updated: 03/06/2015, Legal effective date: 01/02/2012 EMEA/CHMP/EWP/192217/2009 Rev.1 Corr.2
8. ICH guideline M10 on bioanalytical method validation (draft version 25/07/2022); European Medicines Agency, EMA/CHMP/ICH/172948/2019.
9. FDA: Guidance for Industry, Bioanalytical Method Validation. US Food and Drug Administration, 2018.
10. Kuang B, King L, Wang HF. Therapeutic monoclonal antibody concentration monitoring: free or total? Bioanalysis. 2010 Jun;2(6):1125-40. doi: 10.4155/bio.10.64.
11. The Antibody Society, Antibody therapeutics product data, http://www.antibodysociety.org/antibody-therapeutics-product-data (Access at: December 1, 2023)
12. Sailstad JM, Amaravadi L, Clements-Egan A, Gorovits B, Myler HA, Pillutla RC, Pursuhothama S, Putman M, Rose MK, Sonehara K, Tang L, Wustner JT; Global Bioanalysis Consortium. A white paper–consensus and recommendations of a global harmonization team on assessing the Impact of immunogenicity on pharmacokinetic measurements. AAPS J. 2014 May;16(3):488-98. doi: 10.1208/s12248-014-9582-y.
13. Dudal S, Baltrukonis D, Crisino R, Goyal MJ, Joyce A, Osterlund K, Smeraglia J, Taniguchi Y, Yang J. Assay formats: Recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J. 2014 Mar;16(2):194-205. doi: 10.1208/s12248-013-9552-9.

If you are interested in our approach to PK assay, please do not hesitate to contact us. Our scientists consider the biological properties of mAbs, the requirements for its intended application (predicted therapeutic area), and the customer needs to develop a suitable bioassay for PK assessment. Therefore, several techniques for developing the quantitative assay are available at our facility, including ligand binding assays (LBAs) and liquid chromatography-mass spectrometry (LC-MS/MS). For LBAs, we use an enzyme-linked immunosorbent assay (ELISA) or an automated fluorescent immunoassay on the Gyrolab XPlore platform. Click here to obtain more information on our bioanalytics services.
Related resources
Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing

Navigating OOX Results: Effective Analysis and Management in CDMO Laboratories
Analytics, Manufacturing, Regulatory

Mammalian cell lines as a platform for protein vaccine production
Cell culture, Drug substance, Manufacturing, Proteins, Vaccines