Revolution in the EU pharmaceutical legislation ahead
EMA, Regulatory
- The European Commission is planning to revise the existing general pharmaceutical EU legislation. Revisions are hoped to overcome the emergent medical problems such as frequent shortages of critical medicines, unequal access to innovative therapies and antimicrobial drug resistance.
- The proposed changes include reduction of regulatory protection for medicinal products, improved safeguarding of medicines availability across the Europe, introduction of transferable vouchers for antimicrobials, increase in EMA’s financial transparency, streamlining of regulatory procedures and reduction of the environmental impact.
- Whereas the proposed legislation is highly welcomed by practitioners and patients as well as manufacturers of generic (and biosimilar) drugs, companies developing innovative molecules will have to revise their business plans, possibly abandoning the more risky and less viable projects.
- The time of introduction of the new EU pharmaceutical legislation is yet uncertain, as the propositions of European Commission are in conflict with the views of European Parliament.
In April 2023, European Commission adopted a proposal for revised pharmaceutical legislation, set to exert a profound impact on the entire pharmaceutical industry. This planned revision would supersede the existing EU regulations that have fostered the availability of safe and effective medicines for the past two decades (Regulation 726/2004 and Directive 2001/83/EC). The proposal tackles several important medical issues that have surfaced in recent decades, and encompass the following key aspects:
- Reduction in the regulatory protection periods and incentives for both non-orphan and orphan drugs, with the extension option for those that meet the criteria of unmet need, indication expansion or planned launch across the entire EU within 2 years. Currently, non-orphan medicines benefit from 8-year period of data exclusivity and 10-year period of market exclusivity. According to the new EU legislation that timeframe would be reduced to 6 and 8 years, respectively (a reduction of 2 years). Most of the orphan drugs would be less impacted, with only 1-year decrease of data exclusivity for standard ones and no change for products that fulfill the criterion of high unmet medical need. The shortening of regulatory protection period would obviously accelerate the development and approval of generics and biosimilars, therefore achieving broader and more affordable access to novel drugs.
- Improvement in safeguarding of medicines availability through enhanced monitoring and more timely reporting of shortages as well as temporary emergency approvals. Suspension of data and market protection upon emergency approvals is also being considered.
- Introduction of exclusivity voucher for antimicrobials, which can be transferred to another pharmaceutical company. The voucher would offer additional 12 months of regulatory data protection for the drug developer, provided that the antimicrobial in question represents a new therapeutic class, its mechanism of action is completely different from earlier medications and its active substance has not been previously authorized in the EU as a product that treats multi-drug resistant or a serious/life-threatening bacterial infection. Exclusivity vouchers would be introduced as a part of international efforts to tackle antimicrobial resistance (AMR). The EU also plans to promote the best treatment practice, which assumes restricted use of antibiotics, limited to clinically justified cases.
- Streamlining the regulatory processes and activities of EMA committees. The proposal calls for reducing the marketing authorization procedure from 210 down to 180 days and introducing the concept of regulatory sandboxes, which allow for testing innovative technologies, products and services in a restricted and highly supervised real-world environment. Another idea is to allow EMA to introduce label updates according to the available data independently from the marketing authorization holders.
- Strengthening environmental regulations as a part of the EU green transformation plan, for example by increasing the environmental risk assessment (ERA) requirements and optimizing the use of certain medications group (incl. antimicrobials).
- Increasing transparency of procedures, regulatory decisions and interactions between EMA and pharmaceutical industry.
The commission states several objectives of the new pharmaceutical regulations, which together would create a beneficial environment for patients, doctors and the entire pharmaceutical sector:
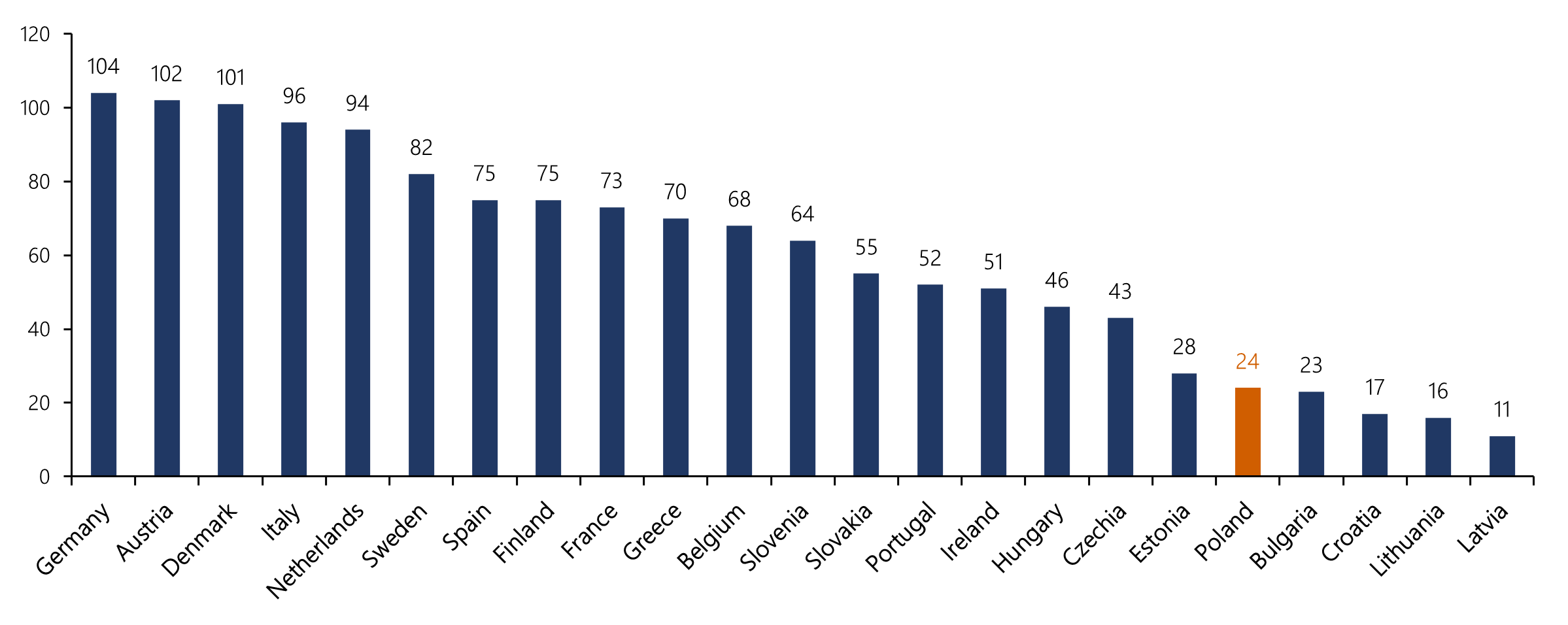
- Ensure timely and equitable access to effective, safe and affordable medications for all patients across the EU, regardless of the place of living. Despite the existence of free trade area between each of the member countries, the access to newly approved drugs is extremely unequal (see figure below). Poland is on the fifth place from the end (24 medicines), much below certain neighboring countries such as Germany (104) or Czechia (43).
- Prevent shortages of medications, which tend to occur more and more frequently in recent years, particularly in Central European countries.
- Continue to offer an attractive and innovation-friendly environment for research, development, and manufacturing of drugs in the EU (through e.g. shortening of regulatory procedures).
- Reduce the environmental footprint of medicines.
- Address antimicrobial resistance, which are predicted to contribute to as many as 10 million deaths per year in 2050, if no preventive measures are introduced.
New EU pharmaceutical legislation
European Commission has comprehensively analyzed the proposed regulations from public health, economic and environmental perspectives, and the results of this analyses were enclosed in special reports. The new EU legislation was also open to public consultation for different stakeholders including governments business representatives, medical professionals and foundations. Hundreds of opinions were submitted, with majority expressing being positive about the proposed changes. The regulatory revision was particularly well received by public institutions and patient organizations. Predictably, several propositions were panned by the spokespersons of pharmaceutical industry. Of all revisions, the reduced period of regulatory protection received the sharpest criticism. It is clear that the consequence of 2-year reduction in market exclusivity may be that the companies will see less commercial value in developing certain innovative drugs and will be forced to abandon the more risky projects. Re-evaluation of benefit-cost ratio of many development programs will be required, particularly for orphan drugs, which even now are frequently unviable. On the other hand, the earlier expiry of regulatory protection is highly welcomed by the manufacturers of generic drugs and biosimilars. Thus, the fallout of the new regulations could be slight tilt of the industry towards the generic drug and biosimilar market.
Unexpectedly, the transferable vouchers for antibiotics are also receiving sharp criticism from industry representatives, who claim that this method of reducing bacterial resistance will likely prove ineffective, and clamor for the entire regulation to be discarded. They bring up multiple arguments, e.g. that such incentives would not be sufficient to make companies return to the antibiotic business. In addition, the critics warn about the potential side effects, which would contradict the entire intention of new regulations, including the potential increase in prices.

Despite many consultations, internal assessments and negotiations between key stakeholders, the revision of pharmaceutical law is still a matter of distant future. In October 2023, the European Parliament (EP) proposed their own revisions with diverging stance on several matters. The EP revisions seem to be more propitious for the pharma industry with respect to the several issues, as they include, among others:
- Extended regulatory protection periods for non-orphan medications with more inclusive definition of unmet need.
- Limited applicability of data and market protection suspensions in case of public health emergency.
- Product label changes cannot be introduced without the consultation with MA holder.
- Softer enforcement of environmental measures, including no automatic refusal of marketing authorization in case of negative ERA outcomes.
So what are the next steps? The conflicting proposals of EC and EP are poised to instigate further revisions and consultations, a course of action that is likely to extend overall several months before the final version is ratified. Although the proposed regulations are probably not going to be enforced any soon, pharma companies must start to proactively adjust their strategies and take into account the new circumstances when planning the development of new molecules. Particularly concerning, in terms of financial sustainability, is the reduced data and market protection, which will translate into much earlier introduction of generic (or biosimilar) products. However, the good news is that the new regulations will certainly prove beneficial for patients, ensuring increased, sustained and more equitable access to innovative drugs and possibly a decreased prevalence of antibiotic-resistant bacteria.
Prepared by:

Adam Tuszyner
Registration Dossier Specialist, Pharmacovigilance Specialist
Literature
1. European Commission. “Reform of the EU pharmaceutical legislation” (April 26, 2023). Link: https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe/reform-eu-pharmaceutical-legislation_en.
2. European Parliament. “Draft Report on the proposal for a directive of the European Parliament and of the Council on the Union code relating to medicinal products for human use, and repealing Directive 2001/83/EC and Directive 2009/35/EC”. Link: https://www.europarl.europa.eu/doceo/document/ENVI-PR-753470_EN.pdf.
3. European Parliament. “Draft Report on the proposal for a regulation of the European Parliament and of the Council laying down Union procedures for the authorization and supervision of medicinal products for human use and establishing rules governing the
European Medicines Agency, amending Regulation (EC) No 1394/2007 and Regulation (EU) No 536/2014 and repealing Regulation (EC) No 726/2004, Regulation (EC) No 141/2000 and Regulation (EC) No 1901/2006”. Link: https://www.europarl.europa.eu/doceo/document/ENVI-PR-753550_EN.pdf.
4. European Commission. “Reform of the EU pharmaceutical legislation: Affordable, accessible and innovative medicines”. Link: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/promoting-our-european-way-life/european-health-union/reform-eu-pharmaceutical-legislation_en.
5. European Commission. “Study in support of the evaluation and impact assessment of the EU pharmaceutical legislation. Evaluation Report.” (June 2022).
6. European Commission. “Study in support of the evaluation and impact assessment of the EU pharmaceutical legislation. Impact Assessment Report.” (June 2022).
7. Bogaert P., Handy E., Dirkzwager R., Wawrzyniak A., Gevrenova Y. “EU Pharma Legislation Review Series: AMR and Transferable Exclusivity Vouchers” (April 28, 2023). Link: https://www.insideeulifesciences.com/2023/04/28/eu-pharma-legislation-review-series-amr-and-transferable-exclusivity-vouchers/.
8. Zhuleku E., Preinfalk F. “European Union to introduce a transferable exclusivity voucher to incentivize the development of new antimicrobials” Allen & Overy (April 28, 2023). Link: https://www.allenovery.com/en-gb/global/blogs/life-sciences-talk/european-union-to-introduce-a-transferable-exclusivity-voucher-to-incentivize-the-development-of-new-antimicrobials.
9. ReAct: News and opinions. “5 reasons why the European Transferable Exclusivity Voucher proposal should go in the bin” (September 28, 2023). Link: https://www.reactgroup.org/news-and-views/news-and-opinions/2023-2/5-reasons-why-the-european-transferable-exclusivity-voucher-proposal-should-go-in-the-bin/.
Related resources
Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing

Navigating OOX Results: Effective Analysis and Management in CDMO Laboratories
Analytics, Manufacturing, Regulatory

Mammalian cell lines as a platform for protein vaccine production
Cell culture, Drug substance, Manufacturing, Proteins, Vaccines