Impact of immunogenicity on efficacy and safety of biosimilars – importance of ADA and nAb testing during the clinical development
KEY POINTS
- The presence of anti-drug antibodies (ADAs) may have a significant impact on efficacy and safety profile of biotherapeutics, leading sometimes to reduced pharmacological activity and/or acute adverse reactions.
- Higher immunogenicity of a candidate biosimilar product is indicative of important quality differences. For this reason, immunogenicity comparison is considered to be a crucial part of biosimilar drug development, regardless of the relevance of ADA formation for the reference molecule.
- Despite the recent calls to limit the clinical testing of biosimilars, EMA and FDA continue to emphasize the importance of extensive immunogenicity evaluation and it is highly unlikely that this requirement will be lifted any soon.
All protein-based therapeutics are immunogenic to some degree – they can be recognized by the immune system as foreign antigens, leading to the development of anti-drug antibodies. The extent to which the drug products elicit the immune response is extremely variable, ranging from 0% for canakinumab (an IL-1 inhibitor) to as high as 40% in case of infliximab (first TNF-α inhibitor introduced on the market). Table 1 below presents immunogenicity rate for the selected, clinically important monoclonal antibodies. The presence of anti-drug antibodies may exert a significant impact on pharmacokinetics, efficacy and safety of these therapeutics, especially those capable of binding to the CDR region and neutralizing their activity (nAbs).
Table 1. Immunogenicity of the selected monoclonal antibodies of high medical importance.
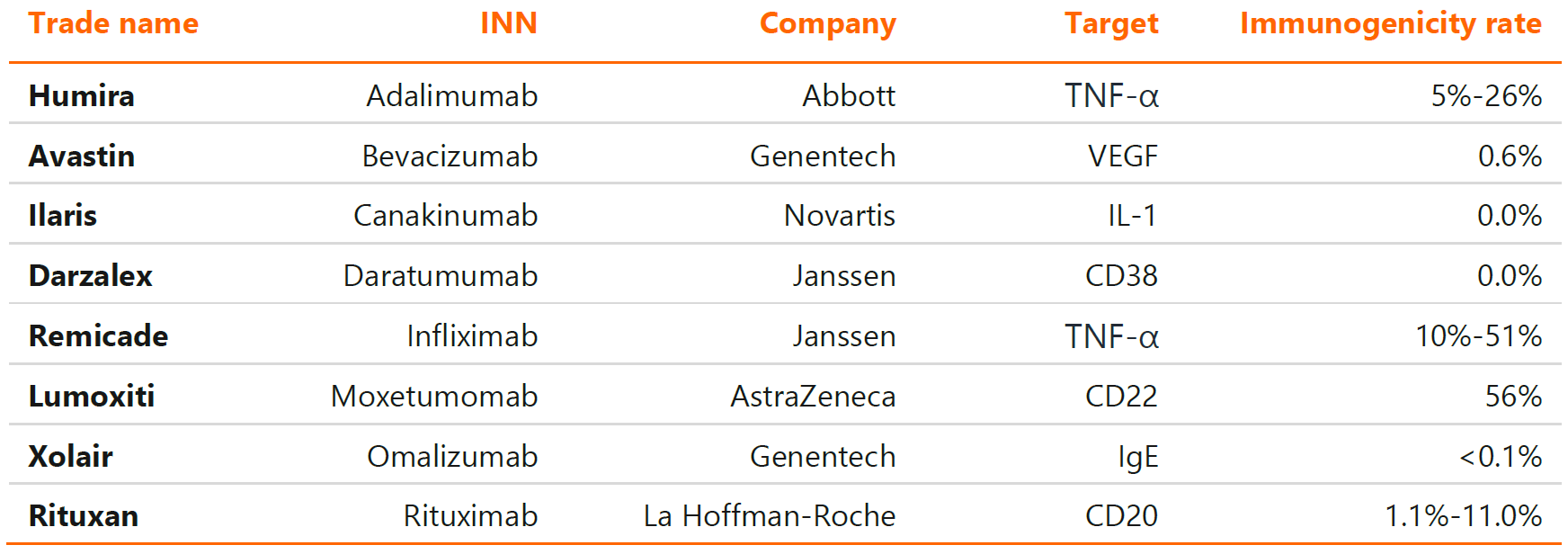
Source: Dingman R., Balu-Iyer S. V., Immunogenicity of protein pharmaceuticals, Journal of Pharmaceutical Sciences, 2019, 108(5): 1637-1654.
In addition to the extreme variability in the immunogenic potential, biologic drugs can differ widely with respect to the clinical consequences of ADA emergence. In case of safety, these may range from no recognizable symptoms to serious anaphylactic reactions, and in case of efficacy from unabated to nearly eliminated therapeutic response. Moreover, in some cases, the administration of biologic drug can induce cross-reactive response to endogenous proteins, a phenomenon, which may lead to the inhibition of vital biochemical processes.
Immunogenicity of innovative biologics
An excellent demonstration of detrimental impact of ADA on the efficacy of innovative biologic is adalimumab, a widely used anti-TNF-α monoclonal antibody. High concentration of ADAs following adalimumab administration is associated with decreased and shortened duration of anti-inflammatory activity, which is indirectly caused by its faster clearance (see Figure 1 below). A good example of adverse impact on safety outcomes comes from another member of TNF-α inhibitor class, infliximab, in which case the ADA emergence may induce severe acute infusion reactions as well as delayed symptoms such as arthralgias, rash, facial oedema or headache. However, the most famous example of safety hazards associated with excessive immune response to biologics is life-threatening pure red cell aplasia, which was caused by recombinant erythropoietin therapy in late 90’s.
Figure 1. Impact of ADA response on pharmacokinetics (left) and efficacy (right) of adalimumab in rheumatoid arthritis.
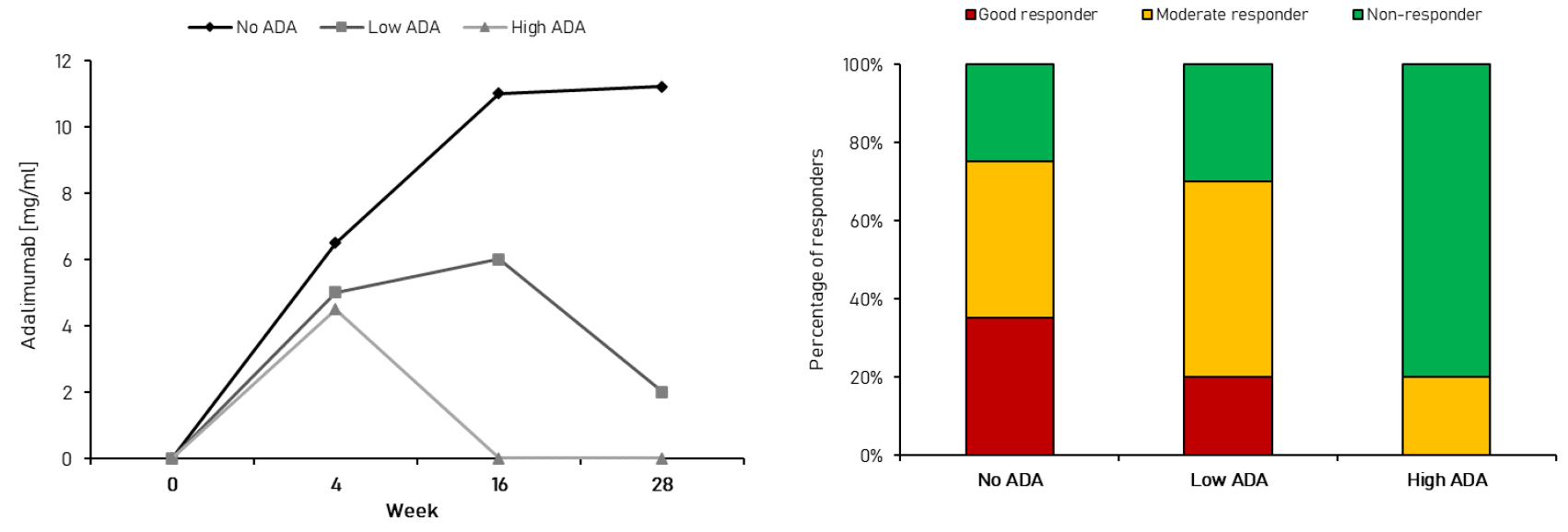
Source: Jiskoot W., Rispens T., Kijanka G., Immunogenicity of Therapeutic Proteins. [in:] Crommelin D., Sindelar R., Meibohm B. (eds.), Pharmaceutical Biotechnology, Springer, Cham, 2019, 139-150.
In stark contrast to the above examples, the immune response to some biotherapeutics, such as rituximab (an anti-CD20 antibody), has little or no influence on efficacy or safety profile. This is despite the fact, that administration of this molecule induces ADA response in up to 11.0% of patients.
Because the immunogenicity of a biologic product can pose serious threat to patients’ safety, regulatory agencies require all biosimilars to undergo clinical evaluation of immunogenicity. This situation is much different and more complicated for innovative biologics. As stated in the EMA’s scientific guideline “Immunogenicity assessment of biotechnology-derived therapeutic proteins”:


Depending on the immunogenic therapeutic protein and/or the rarity of the disease, the extent of immunogenicity data before approval of novel biologic drugs might be limited.


For example, the waiver for ADA assessment can be obtained for some insulin products. As per the FDA guidance “Clinical immunogenicity considerations for biosimilar and interchangeable insulin products”:


“(…) if a comparative analytical assessment based on state-of-the-art technology supports a demonstration of “highly similar” for a proposed biosimilar or interchangeable insulin product, there would be little or no residual uncertainty regarding immunogenicity; in such instances, the proposed biosimilar or interchangeable insulin product, like the reference product, would be expected to have minimal or no risk of clinical impact from immunogenicity. In such instances, a comparative clinical immunogenicity study generally would be unnecessary to support a demonstration of biosimilarity or interchangeability”


Figure 2. Overview of the mechanism of anti-drug immune response.
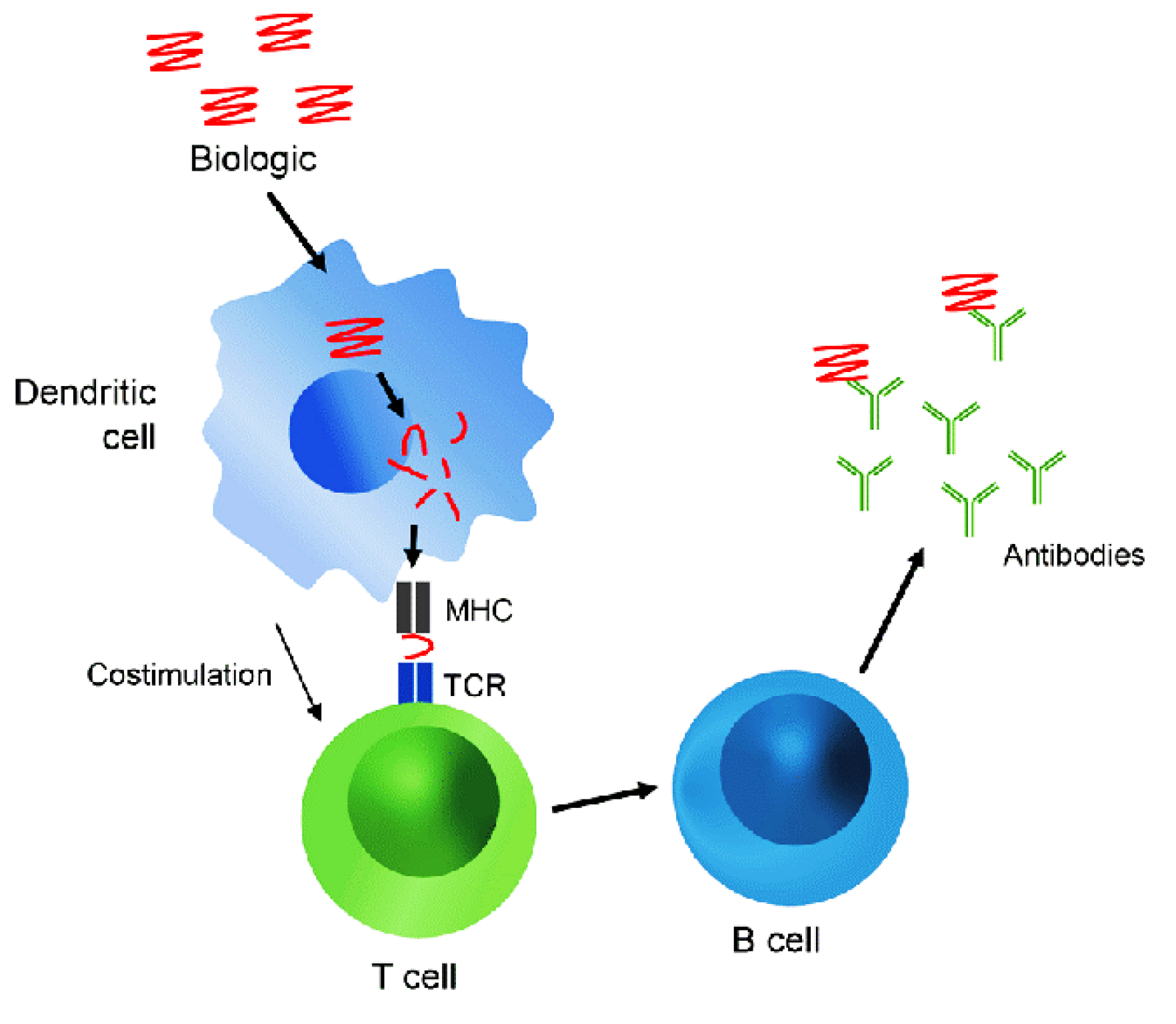
Source: Carr W. W., Jain N., Sublett J. W., “Immunogenicity of botulinum toxin formulations: potential therapeutic implications, Advances in Therapy, 2021, 38(10): 5046-5064.
Immunogenicity of biosimilars
The importance of immunogenicity testing is unequivocal for biosimilars, where higher ADA positivity in comparison to the reference drug may point to important quality differences. Immunogenicity of the therapeutic proteins is determined by multiple different factors, including structural dissimilarities, impurities, formulation, administration route as well as patient features. As biosimilars (by definition) must have the same administration route and target population as the reference drugs, only the first three factors are of concern during the biosimilar product development. Differences in protein glycosylation, oxidation and/or aggregate content are among the most critical attributes capable of triggering the immune response.
Analytical studies of all quality attributes that influence the immunogenicity of biologic drugs are included among Mabion services related to the drug characterization and release testing. Click here for more details.
Nevertheless, up to this day, no major increases in ADA positivity rate have been ever noted for biosimilars compared to the reference drugs, despite so many products having been tested and approved across the world. Interestingly, the opposite situation, that is lower immunogenicity of a biosimilar, has been observed for some authorized products e.g., biosimilars to etanercept (anti-TNF-α fusion protein).
The paucity of cases of increased ADA response is not unexpected given that the major quality attributes influencing immunogenicity are always evaluated as a key part of similarity assessment. Under such development scheme, the situation in which higher ADA response is observed for a biosimilar product without the prior detection of quality issues is unlikely to be encountered. A head-to-head clinical immunogenicity testing based on a fully-validated, tiered approach that includes four main assessment stages: ADA screening, ADA confirmation assays, ADA characterization and titration, and assessment of neutralizing capacity, is critical for the demonstration of clinical similarity and thus regulatory approval. All of these stages may involve a wide variety of bioanalytical platforms, each of which has relative strengths and weaknesses. Mabion offers novel “drug-tolerant” methods including well recognized, fast and highly sensitive methods based on Gyrolab platform as well as ADCC cell-based reporter assay that offers better insight into the functional and biological activity of the drug.
Regulatory perspective
The US and EU regulatory agencies emphasize the importance of immunogenicity evaluation for both innovative and biosimilar biologics. The key guidelines discussing this topic are:
- FDA: “Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products”, 2014.
- EMA: “Immunogenicity assessment of biotechnology-derived therapeutic proteins – Scientific guideline”, Revision 1, 2017.
Both EMA and FDA recommend thorough risk assessment to justify the extent of immunogenicity evaluation in the clinical trials. However, even if the ADA response is expected to be low and inconsequential, the EU agency states that immunogenicity should be studied with validated, fit-for-purpose assays. The typical evaluation scheme should be two-tiered, that is include screening and confirmatory tests followed by neutralizing antibody detection.
In summary, the proper immunogenicity assessment remains an important part of the biosimilar drug development, regardless of the properties of the reference product. The methods for evaluating biologic medicines for ADA are addressed by EU and US regulatory agencies in the dedicated guidelines. In general, a head-to-head, tiered approach involving ADA screening, confirmatory and titration assays followed by neutralizing antibody assessment is required to demonstrate clinical similarity with regards to immunogenicity.
Adam Tuszyner
Literature
- 1. Dingman, Robert, and Sathy V. Balu-Iyer. “Immunogenicity of protein pharmaceuticals.” Journal of pharmaceutical sciences 108.5 (2019): 1637-1654.
- 2. Niazi S. Scientific Rationale for Waiving Clinical Efficacy Testing of Biosimilars. Drug Des Devel Ther. 2022;16:2803-2815 https://doi.org/10.2147/DDDT.S378813
- 3. Han PD, Cohen RD. Managing immunogenic responses to infliximab: treatment implications for patients with Crohn’s disease. Drugs. 2004;64(16):1767-77. doi: 10.2165/00003495-200464160-00004. PMID: 15301561.
- 4. de Oliveira Ascef, Bruna, et al. “Therapeutic Equivalence of Biosimilar and Reference Biologic Drugs in Rheumatoid Arthritis: A Systematic Review and Meta-analysis.” JAMA Network Open 6.5 (2023): e2315872-e2315872.
- 5. Alison Smith, Hugh Manoli, Stacey Jaw, Kimberley Frutoz, Alan L. Epstein, Leslie A. Khawli, Frank-Peter Theil, “Unraveling the Effect of Immunogenicity on the PK/PD, Efficacy, and Safety of Therapeutic Proteins”, Journal of Immunology Research, vol. 2016, Article ID 2342187, 9 pages, 2016. https://doi.org/10.1155/2016/2342187
- 6. Sauna, Zuben E., et al. “Evaluating and mitigating the immunogenicity of therapeutic proteins.” Trends in biotechnology 36.10 (2018): 1068-1084.
- 7. Parikh, Chinar R., et al. “Impact of immunogenicity on clinical efficacy and toxicity profile of biologic agents used for treatment of inflammatory arthritis in children compared to adults.” Therapeutic Advances in Musculoskeletal Disease 13 (2021): 1759720X211002685.
- 8. Niazi, Sarfaraz K. “Rationalizing FDA guidance on biosimilars–expediting approvals and acceptance.” Generics and Biosimilars Initiative Journal (GaBI Journal) 7.2 (2018): 84-91.
- 9. Bennett, Charles L., et al. “Pure red-cell aplasia and epoetin therapy.” New England Journal of Medicine 351.14 (2004): 1403-1408.
- 10. European Medicines Agency (EMA) “Immunogenicity assessment of biotechnology-derived therapeutic proteins – Scientific guideline”, Revision 1, 2017.
- 11. Food and Drug Administration (FDA) “Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products”, August 2014.
- 12. Food and Drug Administration (FDA). “Clinical immunogenicity considerations for biosimilar and interchangeable insulin products.”