Single-Dose Monoclonal Antibody Therapy Offers Durable Protection Against Malaria
- A landmark study published in Nature Medicine reveals that a single administration of the monoclonal antibody CIS43LS provides sustained protection against Plasmodium falciparum, the deadliest species of Plasmodium that causes malaria in humans.
- Phase 2 clinical trial participants receiving CIS43LS exhibited an 87% reduction in malaria incidence over 6 months compared to controls.
Monoclonal Antibody in Malaria Prevention
Research into monoclonal antibodies for malaria prevention continues to evolve, with new candidates being developed and tested. Complementary tools to existing malaria vaccines like RTS, S/AS01 and R21/Matrix-M have shown moderate efficacy, but mAbs offer immediate protection and can be particularly useful in populations that do not respond such well to vaccines.
The advancement of therapies for malaria has been supported by collaborations between research institutions, governments, and philanthropic organizations. The World Health Organization (WHO) has outlined preferred product characteristics for mAbs, emphasizing the need for long-lasting protection and suitability for use in infants and children. Funding from entities like the Bill & Melinda Gates Foundation has been instrumental in driving research and development efforts. These partnerships have facilitated clinical trials and the establishment of biopharma capabilities, laying the groundwork for the potential large-scale manufacturing.
Mechanism of Action
CIS43LS is a human monoclonal antibody engineered for malaria prophylaxis by targeting the circumsporozoite protein (CSP) of Plasmodium falciparum, the species responsible for the majority of malaria-associated mortality. Structurally, it belongs to the IgG1 subclass and includes Fc domain modifications (M428L and N434S, or “LS” mutations). That mutations allowing for extended bioavailability and maintaining protective titers across a 6-month period.
The antibody’s epitope recognition is highly specific: CIS43LS binds to a conserved region of the central repeat domain of CSP, notably the junctional sequence between the N-terminal domain and the NANP repeat region. This region is essential for sporozoite motility and hepatocyte invasion, making it a critical vulnerability point for immunological intervention.
Upon entry of the malaria-infected mosquito’s sporozoites into the human host, CIS43LS intercepts the parasites in the bloodstream before they reach the liver. By binding to CSP with high affinity, the antibody sterically hinders the sporozoite’s traversal through the sinusoidal endothelial barrier and prevents recognition of hepatocyte surface receptors necessary for cellular invasion.
This neutralizing mechanism effectively halts the parasite’s life cycle at its earliest stage, preventing the development of hepatic schizonts and, consequently, the downstream erythrocytic cycle that causes clinical symptoms and transmission. In this way, CIS43LS not only protects the individual recipient but may also reduce community-level transmission if deployed broadly.
Its prolonged circulation and single-dose efficacy enable long-term prophylaxis, especially valuable in seasonal transmission zones. The targeted action at a conserved epitope also suggests resilience against antigenic variation, a common challenge in malaria vaccine design. With its minimal requirement for downstream immune activation, CIS43LS is poised to fill critical gaps in malaria control.
Clinical Trial Results
The fight against malaria took a promising turn with a Phase 2 clinical trial NCT04329104 evaluating the efficacy of CIS43LS, a monoclonal antibody designed to neutralize Plasmodium falciparum sporozoites. Researchers aimed to determine if a single intravenous dose could confer long-lasting protection during Mali’s intense malaria transmission season.
The study enrolled 330 healthy adults between 18 and 55 years of age selected from a group of 742 adults based on eligibility criteria, who were randomized into three groups: one receiving 10 mg/kg of CIS43LS, another receiving 40 mg/kg, and the third receiving a placebo. To ensure a clear assessment of the antibody’s efficacy, all participants were administered artemether-lumefantrine prior to the infusion to clear any existing infections.
Participants were then monitored over a 6-month period, with regular assessments using both thick blood smear microscopy and a highly sensitive qRT-PCR assay. This comprehensive monitoring strategy allowed for the detection of new infections, providing critical data on the duration and extent of protection offered by CIS43LS.
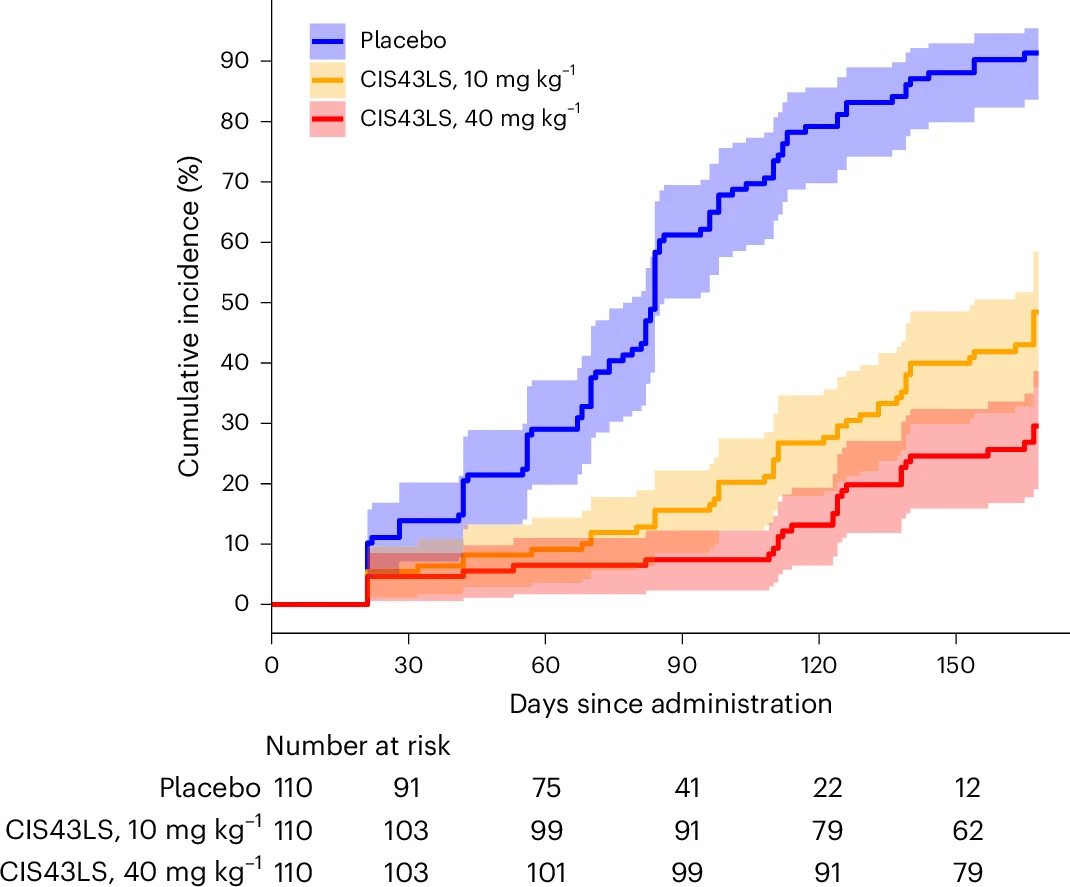
Figure 1. Cumulative incidence of P. falciparum blood-stage infection during a 6-month malaria season after a single IV infusion of CIS43LS (10 mg/kg or 40 mg/kg; n=110 each) or placebo (n=110). Infections were identified via 18S qRT-PCR from dried blood spots (DBS) collected at scheduled and illness-triggered visits. Sampling occurred pre-dose (day 0), on days 1, 3, 7, 14, 21, 28, and biweekly until day 168.
This trial revealed a critical gap between traditional microscopy and molecular diagnostics in evaluating malaria prevention. Of 5,015 dried blood spots (DBS) analyzed across the study, 838 were qRT-PCR positive, yet only 232 were detectable by TBS demonstrating that more than two-thirds of low-density infections go undetected with conventional tools. Using the highly sensitive 18S rRNA qRT-PCR assay – capable of detecting as few as 20 parasites/mL – researchers identified a strong protective effect from a single intravenous dose of the monoclonal antibody.
The antibody’s efficacy against gametocytemia was calculated at 87.7% for the 40 mg/kg dose (95% CI, 75.6–93.8%) and 73.0% for the 10 mg/kg dose (95% CI, 54.0–84.0%), with both comparisons reaching strong statistical significance. These findings suggest that CIS43LS does not merely protect individuals from clinical malaria, but also reduces the parasite reservoir available to sustain community-level transmission.
Next Steps in Monoclonal Antibody Development
Following the promising Phase 2 trial results, the next logical step for CIS43LS is the initiation of a pivotal Phase 3 clinical study in diverse malaria-endemic populations. This study should examine repeat-dose scenarios to assess long-term protection and seasonal re-administration feasibility.
Given the urgency of malaria prevention tools, a sponsor may consider pursuing accelerated regulatory pathways such as Emergency Use Authorization (EUA), WHO prequalification, or regional priority review mechanisms. These programs often require early engagement with regulators and rapid generation of CMC data to support submission.
Parallel to clinical development, a sponsor should begin process characterization and scale-up of the CIS43LS manufacturing platform. At this stage, choosing a biologics CDMO with deep expertise in mammalian expression systems, upstream/downstream optimization, and robust tech transfer capabilities is critical to ensure consistency and regulatory readiness. The GMP Drug Substance (DS) manufacturing process must transition from pilot to commercial scale. This involves optimizing upstream (cell line development) and downstream (purification) bioprocessing steps for consistent yield, quality, and cost-efficiency. Process development should include identification of critical process parameters (CPPs) and control strategies using a quality-by-design (QbD) approach.
In addition to CIS43LS, researchers are developing L9LS, another monoclonal antibody targeting a different epitope on CSP. L9LS was designed for subcutaneous administration, offering a more practical delivery method, especially in resource-limited settings. In a Phase 2 clinical trial involving children aged 6 to 10 years in Mali, L9LS showed 77% efficacy in preventing symptomatic malaria over a 6-month period.
Prepared by:

Jakub Knurek
Marketing Specialist
Sources and further reading
- Skinner J, Kayentao K, Ongoiba A, Healy SA, Hu Z, Preston AC, Niangaly A, Schwabl P, Cisse H, Doumbo S, Doumtabe D, Traore A, Li S, Peterson ME, Seilie AM, Chavtur C, Staubus W, Chang M, Kelley K, Traore H, Djiguiba A, Keita M, Ouattara A, Doucoure M, Keita M, Diarra D, Sylla M, Diakite D, Konate M, Traore S, Zéguimé A, Dolo A, Neafsey DE, Murphy SC, Traore B, Seder RA, Crompton PD. Anti-sporozoite monoclonal antibody for malaria prevention: secondary efficacy outcome of a phase 2 randomized trial. Nat Med. 2025 Jun 3.
- Kisalu NK, Silva Pereira LD, Herman JD, Asokan M, Ernste K, Merriam J, Liu C, DeMouth ME, Pegu A, Lofgren M, Dillon M, Bonilla B, MacVicar R, Zur Y, Kiyuka P, Flores-Garcia Y, Chakraborty S, Nikolaeva D, Ogwang R, Flynn B, Francica J, Pierson TC, Koup RA, Zavala F, Wang TT, Alter G, Idris AH, Seder RA. FcγR binding differentially contributes to protection by two human monoclonal antibodies targeting Plasmodium falciparum circumsporozoite protein. Sci Transl Med. 2025; 17(795): eadk6745.
- Kayentao K, Ongoiba A, Preston AC, Healy SA, Hu Z, Skinner J, Doumbo S, Wang J, Cisse H, Doumtabe D, Traore A, Traore H, Djiguiba A, Li S, Peterson ME, Telscher S, Idris AH, Adams WC, McDermott AB, Narpala S, Lin BC, Serebryannyy L, Hickman SP, McDougal AJ, Vazquez S, Reiber M, Stein JA, Gall JG, Carlton K, Schwabl P, Traore S, Keita M, Zéguimé A, Ouattara A, Doucoure M, Dolo A, Murphy SC, Neafsey DE, Portugal S, Djimdé A, Traore B, Seder RA, Crompton PD; Mali Malaria mAb Trial Team. Subcutaneous Administration of a Monoclonal Antibody to Prevent Malaria. N Engl J Med. 2024; 390(17): 1549-1559.
- Lyke KE, Berry AA, Mason K, Idris AH, O’Callahan M, Happe M, Strom L, Berkowitz NM, Guech M, Hu Z, Castro M, Basappa M, Wang L, Low K, Holman LA, Mendoza F, Gordon IJ, Plummer SH, Trofymenko O, Strauss KS, Joshi S, Shrestha B, Adams M, Chagas AC, Murphy JR, Stein J, Hickman S, McDougal A, Lin B, Narpala SR, Vazquez S, Serebryannyy L, McDermott A, Gaudinski MR, Capparelli EV, Coates EE, Wu RL, Ledgerwood JE, Dropulic LK, Seder RA; VRC 612 Part C Study Team. Low-dose intravenous and subcutaneous CIS43LS monoclonal antibody for protection against malaria (VRC 612 Part C): a phase 1, adaptive trial. Lancet Infect Dis. 2023; 23(5): 578-588.
- Kayentao K, Ongoiba A, Preston AC, Healy SA, Doumbo S, Doumtabe D, Traore A, Traore H, Djiguiba A, Li S, Peterson ME, Telscher S, Idris AH, Kisalu NK, Carlton K, Serebryannyy L, Narpala S, McDermott AB, Gaudinski M, Traore S, Cisse H, Keita M, Skinner J, Hu Z, Zéguimé A, Ouattara A, Doucoure M, Dolo A, Djimdé A, Traore B, Seder RA, Crompton PD; Mali Malaria mAb Trial Team. Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali. N Engl J Med. 2022; 387(20): 1833-1842.
- Lab-made antibody stops malaria. Nat Biotechnol. 2022; 40: 1304.
- Wu RL, Idris AH, Berkowitz NM, Happe M, Gaudinski MR, Buettner C, Strom L, Awan SF, Holman LA, Mendoza F, Gordon IJ, Hu Z, Campos Chagas A, Wang LT, Da Silva Pereira L, Francica JR, Kisalu NK, Flynn BJ, Shi W, Kong WP, O’Connell S, Plummer SH, Beck A, McDermott A, Narpala SR, Serebryannyy L, Castro M, Silva R, Imam M, Pittman I, Hickman SP, McDougal AJ, Lukoskie AE, Murphy JR, Gall JG, Carlton K, Morgan P, Seo E, Stein JA, Vazquez S, Telscher S, Capparelli EV, Coates EE, Mascola JR, Ledgerwood JE, Dropulic LK, Seder RA; VRC 614 Study Team. Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N Engl J Med. 2022; 387(5): 397-407.
- Gaudinski MR, Berkowitz NM, Idris AH, Coates EE, Holman LA, Mendoza F, Gordon IJ, Plummer SH, Trofymenko O, Hu Z, Campos Chagas A, O’Connell S, Basappa M, Douek N, Narpala SR, Barry CR, Widge AT, Hicks R, Awan SF, Wu RL, Hickman S, Wycuff D, Stein JA, Case C, Evans BP, Carlton K, Gall JG, Vazquez S, Flach B, Chen GL, Francica JR, Flynn BJ, Kisalu NK, Capparelli EV, McDermott A, Mascola JR, Ledgerwood JE, Seder RA; VRC 612 Study Team. A Monoclonal Antibody for Malaria Prevention. N Engl J Med. 2021; 385(9): 803-814.
- Kisalu NK, Pereira LD, Ernste K, Flores-Garcia Y, Idris AH, Asokan M, Dillon M, MacDonald S, Shi W, Chen X, Pegu A, Schön A, Zavala F, Balazs AB, Francica JR, Seder RA. Enhancing durability of CIS43 monoclonal antibody by Fc mutation or AAV delivery for malaria prevention. JCI Insight. 2021; 6(3): e143958.