CHO Cell Lines for Recombinant Protein Production
Drug development, Drug product, Manufacturing
CHO (Chinese Hamster Ovary) cell lines have emerged as a powerful intersection of cellular biology and bioprocess engineering, offering an highly effective expression system for therapeutic proteins. Their unique ability to combine scalability with clinically acceptable glycosylation profiles has transformed them into the industry’s dominant production platform. But as biologics become more advanced, so must the technologies and strategies that support CHO cell line development.
CHO Cell Lines in Biomanufacturingacturing
CHO cell lines have emerged as the most extensively used expression system in the biopharmaceutical industry, responsible for producing the majority of currently approved therapeutic proteins (almost 90%).1 Their unique cellular characteristics, and scalability have positioned them as the cornerstone of modern biomanufacturing processes for complex biologics.
At the core of CHO cells’ suitability is their ability to perform human-like post-translational modifications (PTMs), particularly N-linked glycosylation, that closely resemble those found in human proteins.2,3 This is essential for the biological activity, half-life, and immunogenicity profile of many protein-based therapeutics, including monoclonal antibodies, enzymes, and vaccine antigens. While CHO cells do not replicate all human glycosylation patterns, their PTM profiles are sufficient for the vast majority of approved biotherapeutics, and glycoengineering advances continue to close the remaining gaps.4
The biomanufacturing utility of CHO cells is also underpinned by their adaptability to serum-free and chemically defined suspension cultures. This allows high-density cultivation in bioreactors, supporting volumetric productivity at industrial scales. Under optimized fed-batch conditions, CHO cultures have ability to achieve titers ranging from 3-5 g/L, and in some cases, even higher.5 Such productivity levels meet the demands of global therapeutic markets while maintaining cost-efficiency in large-scale manufacturing.
Optimization Strategies for CHO Cell Line Development
CHO host selection is about finding the right balance between cellular performance, glycosylation fidelity, scalability, and regulatory compliance, not just about maximizing yield. The path to success begins with a strategic understanding of how CHO subtypes compare under different process conditions.
Although all CHO lines share a common ancestor, decades of mutagenesis and clonal selection have resulted in distinct cell lines with divergent metabolic preferences and genetic architectures.6 The three most common are:
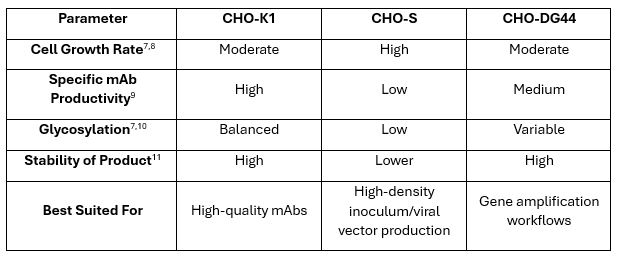
The foundation of any successful cell line development begins with tailored cell line selection. Each CHO lineage has evolved distinct phenotypic characteristics that can be leveraged depending on the product’s specific needs. For example, when the therapeutic objective is to enhance antibody-dependent cellular cytotoxicity (ADCC), CHO-DG44 cells are particularly well-suited.12 These cells tend to produce antibodies with low fucosylation and higher mannose content – two glycan features known to enhance effector function. Conversely, for long-circulating antibodies where improved pharmacokinetics is a priority, CHO-K1 cells may be the preferred host due to their higher natural levels of galactosylation and sialylation, which correlate with extended serum half-life.13 Selecting the right host based on these traits ensures that the cell line’s metabolic and processing machinery aligns with the product’s therapeutic profile and production goals.
Approaches to Enhance Protein Expression
Once the optimal host is selected, media optimization becomes central to sustaining and enhancing protein expression in CHO cells. Even high-performing CHO lines will not reach their full potential without culture conditions that match their unique metabolic and energy requirements. A well-balanced media and/or feed formulation must supply sufficient precursors for mitochondrial energy production, support endoplasmic reticulum (ER) function to ensure efficient protein folding and secretion, and suppress the accumulation of metabolic byproducts such as lactate and ammonia, which can impair cell health and reduce yield.
In parallel, genetic engineering and amplification tools provide powerful levers to the efficiency of protein expression in CHO cells. The use of Bacterial Artificial Chromosomes (BACs) enables stable, position-independent gene integration, avoiding the variability often seen with random plasmid insertion.14 This method ensures high, consistent expression of the therapeutic gene without the need for extensive clonal screening. From a technological perspective, CHO cells are well-suited for genetic manipulation and selection strategies. Common systems, such as DHFR/methotrexate15 and glutamine synthetase/MSX16, enable the amplification of transgenes and the selection of high-producing clones. These approaches are integral to the generation of stable CHO cell lines capable of long-term, consistent protein expression.
Another issue of optimization involves glycosylation control, a critical quality attribute for therapeutic proteins, especially in the development of monoclonal antibodies. Subtle shifts in glycan profiles can dramatically affect efficacy, safety, and immunogenicity.17 By introducing specific culture supplements – such as galactose, manganese, or uridine – producers can steer glycosylation toward desired profiles, increasing the prevalence of functional glycoforms.18 Equally important is the use of real-time analytics to monitor glycosylation trends during scale-up, allowing for early intervention if deviations occur. Selecting host cells with genetically stable glycosylation enzyme pathways further supports the consistent production of proteins that meet strict regulatory requirements for critical quality attributes (CQAs).
Upstream and Downstream Process Considerations in Recombinant Protein Production
Upstream and downstream processes must be co-developed as part of biologics process development, as upstream improvements can shift downstream challenges. An integrated development approach aligns bioreactor operation, feed strategy, and harvest timing with purification capacity and quality expectations.
The upstream phase focuses on cultivating a controlled environment for robust cell growth and optimal recombinant protein production. Temperature, pH, and dissolved oxygen (DO) are key parameters that influence protein folding and glycosylation. Standard CHO cultures run at 36-37°C, with hypothermic shifts to 32°C sometimes applied in later stages to extend culture duration, increase amount of product and improve its quality19. pH is typically controlled between 6.8 and 7.2, while DO between 30-60% saturation, supporting aerobic metabolism while avoiding oxidative stress.20,21 Agitation and aeration must ensure uniform conditions and efficient oxygen transfer while minimizing shear stress. Deviations can impact enzyme function, viability, and overall protein yield.
Downstream processing starts with harvest and clarification, then moves through purification and formulation. The aim is to isolate the protein efficiently while maintaining CQAs such as purity, activity, and glycosylation — all critical to recombinant protein production. Protein A affinity chromatography is standard for monoclonal antibody capture, offering high specificity and yield.22,23 Maintaining glycosylation consistency is a key challenge. Buffer composition and pH control are vital throughout purification to maintain protein structure and ensure efficient separation.
Next-Gen CHO Platforms Development with Higher Productivity and Customized Glycosylation Profile
The shift to next-gen platforms is underpinned by a revolution in omics.24 High-resolution genomics, transcriptomics, proteomics, and epigenomics allow manufacturers to map and understand the full biological “software” running within CHO cells. By dissecting transcriptional bottlenecks, secretory limitations, and regulatory architectures, scientists can rationally design host systems that consistently yield high-expression phenotypes.25 This multi-layered insight is especially powerful when applied to clonal selection. Using specific markers, developers can predict high-performing clones at the single-cell stage.
Cell Line Development platforms are increasingly purpose-built to support the development of advanced therapeutics. Biologics place significant strain on the cell’s folding, glycosylation, and secretion machinery, requiring hosts with expanded ER capacity, optimized chaperone expression, and metabolic pathways designed to sustain high biosynthetic throughput.
Customized glycosylation profiles are now addressed at the cellular level.7 CHO hosts are engineered with precise control over glycosyltransferase expression, substrate availability, and enzymatic competition to ensure glycan patterns align with the target product profile. This improves therapeutic function and ensures batch-to-batch consistency that meets increasingly stringent regulatory expectations.
Quality and Regulatory Considerations for CHO-Based Protein Production
Global regulatory agencies – including the EMA, FDA, and PMDA – maintain clear expectations for CHO-based biologics, particularly around the principles of Quality by Design (QbD), scalable recombinant protein production, consistent manufacturing performance, and product comparability.26
Master Cell Banks (MCB) and Working Cell Banks (WCB) must be fully characterized for identity, purity, and stability. This includes documentation of origin, genetic constructs, and absence of adventitious agents. The source and quality of all raw materials – including serum-free, chemically defined media and supplements – must be documented and controlled to ensure consistency and mitigate contamination risks. Developers are expected to establish a well-justified design space and demonstrate that the process consistently delivers product meeting predefined CQAs. This includes validated Critical Process Parameters (CPPs) and in-process controls.27,28 Any process change, scale-up, or site transfer must be supported by comparability data using sensitive and orthogonal methods that assess structure, function, and glycosylation profiles. Agencies expect manufacturers to tailor risk mitigation strategies based on the complexity of the molecule. For example, bispecific antibodies or Fc-engineered variants may require more stringent analytical characterization and functional assays. Post-approval monitoring plans are essential to ensure continued process control and product quality over time. Real-time data collection and statistical trend analysis are increasingly expected as part of modern CPV programs.
The Future of CHO Cell Lines in Recombinant Protein Manufacturing
Driven by personalized medicine, new methods and global access targets, demand for advanced biologic drugs continues to rise. Emerging therapeutic formats present new challenges that require tailored cellular machinery. As the industry pivots toward speed, sustainability, and modularity, high-throughput and flexible biomanufacturing platforms potential grows.29 CHO cells in biomanufacturing play a central role in meeting these demands. CHO hosts with enhanced energy metabolism and expanded secretory capacity are enabling faster bioprocesses without compromising product quality. Advances in single-use technologies, continuous processing, and integrated biomanufacturing lines are allowing rapid scale-up and scale-out of CHO-based processes. These modular systems are particularly valuable for personalized biologics, rare disease therapeutics, and pandemic response efforts. A CDMO company equipped with such technologies can help biopharma innovators implement these solutions quickly and at scale, ensuring both agility and compliance in modern biologics production.
FAQ
Prepared by:

Karolina Lonkwic
Team Leader, Upstream Manager

Anna Moskal
Team Leader, Process Engineer

Jakub Knurek
Marketing Specialist
References
- Walsh G, Walsh E. Biopharmaceutical benchmarks 2022. Nat Biotechnol. 2022; 40(12): 1722-1760.
- Bryan L, Clynes M, Meleady P. The emerging role of cellular post-translational modifications in modulating growth and productivity of recombinant Chinese hamster ovary cells. Biotechnol Adv. 2021; 49: 107757.
- Donini R, Haslam SM, Kontoravdi C. Glycoengineering Chinese hamster ovary cells: a short history. Biochem Soc Trans. 2021; 49(2): 915-931.
- Bryan L, Clynes M, Meleady P. The emerging role of cellular post-translational modifications in modulating growth and productivity of recombinant Chinese hamster ovary cells. Biotechnol Adv. 2021; 49: 107757.
- Zhou Y, Raju R, Alves C, Gilbert A. Debottlenecking protein secretion and reducing protein aggregation in the cellular host. Curr Opin Biotechnol. 2018; 53: 151-157.
- Wurm FM, Wurm MJ. Cloning of CHO Cells, Productivity and Genetic Stability—A Discussion. Processes. 2017; 5(2): 20.
- Reinhart D, Damjanovic L, Kaisermayer C, Sommeregger W, Gili A, Gasselhuber B, Castan A, Mayrhofer P, Grünwald-Gruber C, Kunert R. Bioprocessing of Recombinant CHO-K1, CHO-DG44, and CHO-S: CHO Expression Hosts Favor Either mAb Production or Biomass Synthesis. Biotechnol J. 2019; 14(3): e1700686.
- Tihanyi B, Nyitray L. Recent advances in CHO cell line development for recombinant protein production. Drug Discov Today Technol. 2020; 38: 25-34.
- Boon L. Which CHO cell is the right CHO cell? Drug Target Review, 2021.
- Kotidis P, Donini R, Arnsdorf J, Hansen AH, Voldborg BGR, Chiang AWT, Haslam SM, Betenbaugh M, Jimenez Del Val I, Lewis NE, Krambeck F, Kontoravdi C. CHOGlycoNET: Comprehensive glycosylation reaction network for CHO cells. Metab Eng. 2023; 76: 87-96.
- Tanemura H, Masuda K, Okumura T, Takagi E, Kajihara D, Kakihara H, Nonaka K, Ushioda R. Development of a stable antibody production system utilizing an Hspa5 promoter in CHO cells. Sci Rep. 2022; 12(1): 7239.
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004; 87(5): 614-622.
- Blundell PA, Lu D, Dell A, Haslam S, Pleass RJ. Choice of Host Cell Line Is Essential for the Functional Glycosylation of the Fc Region of Human IgG1 Inhibitors of Influenza B Viruses. J Immunol. 2020; 204(4): 1022-1034.
- Aga M, Yamano N, Kumamoto T, Frank J, Onitsuka M, Omasa T. Construction of a gene knockout CHO cell line using a simple gene targeting method. BMC Proc. 2015; 9: P2.
- Ng SK. Generation of high-expressing cells by methotrexate amplification of destabilized dihydrofolate reductase selection marker. Methods Mol Biol. 2012; 801: 161-172.
- Tian J, He Q, Oliveira C, Qian Y, Egan S, Xu J, Qian NX, Langsdorf E, Warrack B, Aranibar N, Reily M, Borys M, Li ZJ. Increased MSX level improves biological productivity and production stability in multiple recombinant GS CHO cell lines. Eng Life Sci. 2020; 20: 112-125.
- Gyorgypal A, Fratz-Berilla E, Kohnhorst C, Powers DN, Chundawat SPS. Temporal Galactose-Manganese Feeding in Fed-Batch and Perfusion Bioreactors Modulates UDP-Galactose Pools for Enhanced mAb Glycosylation Homogeneity. Biotechnol Bioeng. 2025.
- Loebrich S, Clark E, Ladd K, Takahashi S, Brousseau A, Kitchener S, Herbst R, Ryll T. Comprehensive manipulation of glycosylation profiles across development scales. MAbs. 2019; 11(2): 335-349.
- Xu J, Tang P, Yongky A, Drew B, Borys MC, Liu S, Li ZJ. Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling. MAbs. 2019; 11(1): 191-204.
- Lee JC, Kim DY, Oh DJ, Chang HN. Long-term operation of depth filter perfusion systems (DFPS) for monoclonal antibody production using recombinant CHO cells: Effect of temperature, pH, and dissolved oxygen. Biotechnol Bioproc E. 2008; 13: 401-409.
- Liang K, Luo H, Li Q. Optimization of the Process of Chinese Hamster Ovary (CHO) Cell Fed-Batch Culture to Stabilize Monoclonal Antibody Production and Overall Quality: Effect of pH Control Strategies. Fermentation 2024; 10(7): 352.
- Grom M, Kozorog M, Caserman S, Pohar A, Likozar B. Protein A affinity chromatography of Chinese hamster ovary (CHO) cell culture broths containing biopharmaceutical monoclonal antibody (mAb): Experiments and mechanistic transport, binding and equilibrium modeling. J Chromatogr B Analyt Technol Biomed Life Sci. 2018; 1083: 44-56.
- Kozorog M, Caserman S, Grom M, Vicente FA, Pohar A, Likozar B. Model-based process optimization for mAb chromatography. Sep. Purif. Technol. 2023; 305: 122528.
- O’Callaghan PM, Di Cara A. Application of Omics Technologies: Creating Next-Generation CHO Expression Platforms. BioProcess Int. 2024.
- Lin D, Yalamanchili HB, Zhang X, Lewis NE, Alves CS, Groot J, Arnsdorf J, Bjørn SP, Wulff T, Voldborg BG, Zhou Y, Zhang B. CHOmics: A web-based tool for multi-omics data analysis and interactive visualization in CHO cell lines. PLoS Comput Biol. 2020; 16(12): e1008498.
- Amann T, Hansen AH, Kol S, Hansen HG, Arnsdorf J, Nallapareddy S, Voldborg B, Lee GM, Andersen MR, Kildegaard HF. Glyco-engineered CHO cell lines producing alpha-1-antitrypsin and C1 esterase inhibitor with fully humanized N-glycosylation profiles. Metab Eng. 2019; 52: 143-152.
- ICH Q5D, Derivation and characterisation of cell substrates used for production of biotechnological/biological products, European Medicines Agency.
- ICH Q5B, Quality of Biotechnological Products: Analysis of the expression construct in cell lines used for production of rDNA-derived protein products, European Medicines Agency.
- Park SY, Choi DH, Song J, Lakshmanan M, Richelle A, Yoon S, Kontoravdi C, Lewis NE, Lee DY. Driving towards digital biomanufacturing by CHO genome-scale models. Trends Biotechnol. 2024; 42(9): 1192-1203.
Related resources

Biologics Characterization for Ensuring Product Quality and Consistency
Analytics, Biologics
Monoclonal Antibody Prophylaxis for Infectious Diseases – Passive Immunization and Prevention
Monoclonal antibody, Vaccines

End-to-End Manufacturing of Biosimilars – From Cell Line to Commercial Product
Biosimilars, Manufacturing