Science News
Discover the news and inspiring materials from the world of biotechnology.

FDA Grants First Ever Waiver of Clinical Efficacy Studies for Monoclonal Antibody Biosimilar
For the first time in history, the FDA has granted an exemption from the requirement to conduct Clinical Efficacy Studies of biosimilar drugs containing monoclonal antibodies, signaling a fundamental shift toward evidence-based approval pathways. This exemption underscores the growing confidence in a regulatory approach based on high quality scientific research, recognizing that robust comparative data can sufficiently demonstrate biosimilarity. The removal of the requirement for Clinical Efficacy Studies represents a historic milestone for the biopharmaceutical sector, reducing the duplication of clinical knowledge already established for reference products.
Learn more
Biologics CDMO Market – Mabion Approach to Establishing Cooperation
The CDMO industry for biologics continues to grow, offering complex solutions for the biopharmaceutical industry. Mabion’s winning formula? You will find the answer in a conversation between Nigel Stapleton and Marty Henehan.
Learn more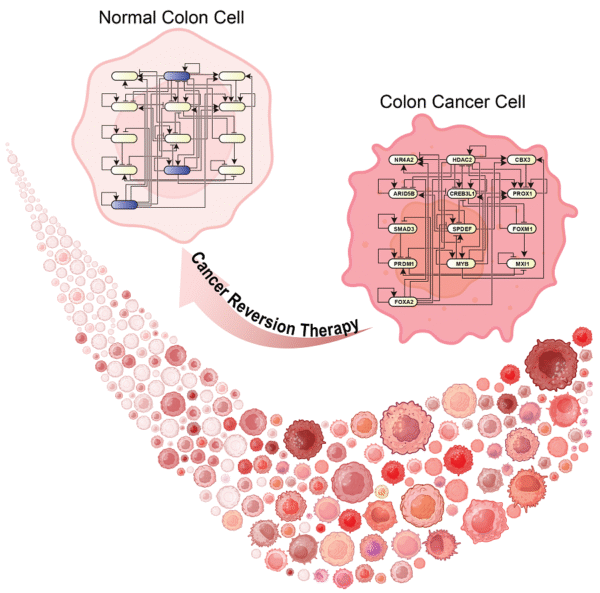
Reprogramming Cancer Cells: A New Paradigm in Colon Cancer Reversion Therapy
Cancer Reversion Therapy is groundbreaking new strategy instead aims to reprogram tumor cells back into normal cells, potentially overcoming drug resistance and side effects associated with cell-killing therapies. Research demonstrates that inhibiting a few key genetic “switches” can induce malignant colon cells to differentiate into benign intestinal cells, effectively reversing their cancerous state.
Learn more
Mabion Launches €500,000 Services Contest to Support Next-Gen Oncology Breakthroughs
At Mabion we understand how to advance promising biosimilars and novel biotherapeutics to the clinic and from there to patients. Unfortunately, many promising biopharmaceutical innovations spanning novel recombinant protein therapeutics to biosimilars are being held back in the current, limiting funding climate, depriving patients from access to advanced therapies. Yet the urgency to close gaps in unmet patient care has never been greater.That is why we are launching the “Unlocking the Future of Therapeutics” competition a unique opportunity to support and accelerate biologics development and scale up for an oncology-targeted therapeutic.We are offering €500,000 credit in development services to support an exceptional therapeutic project. This can be a novel therapeutic, but might also be a biosimilar drug which expands patients access to lifesaving care.We have partnered with respected academic experts focused on cancer research to select the most promising candidate.
Learn more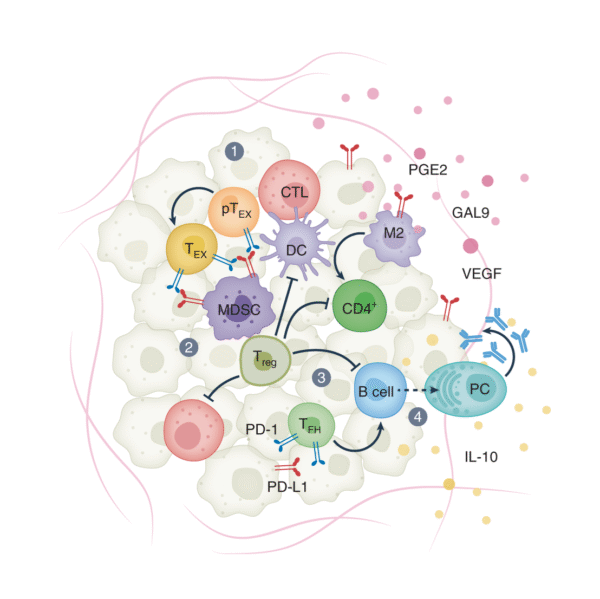
Melanoma Immunotherapy Enhance by Immunity Activation
By reintroducing the Interleukin-4 into IFN-I–dominant, inflammation-deficient tumors, scientists successfully reinvigorated T cell–driven tumor rejection. Whether introduced directly or delivered via engineered T cells in ACT, IL-4 helped reshape the immune landscape, boosting effector T cells and improving survival in mouse models.
Learn more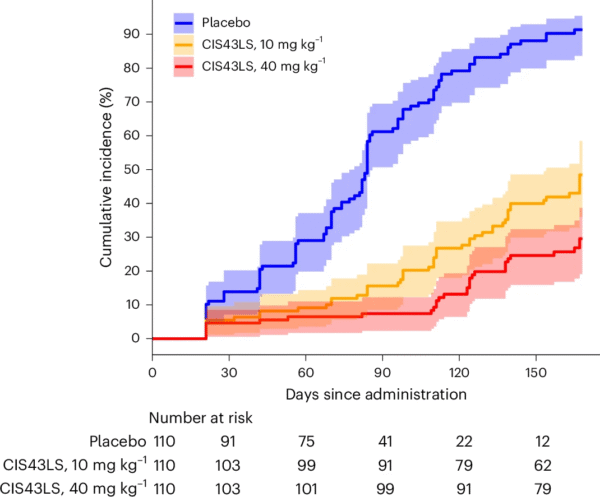
Single-Dose Monoclonal Antibody Therapy Offers Durable Protection Against Malaria
Monoclonal antibody CIS43LS reduced the cumulative incidence of infection by 87.4% at 40 mg/kg during the 6-month qRT-PCR monitoring period. This Phase 2 trial evaluated its efficacy in 330 adults in Mali during a 6-month high-transmission season.CIS43LS is a human IgG1 monoclonal antibody targeting the circumsporozoite protein of Plasmodium falciparum, designed to confer long-acting protection with a single intravenous dose.
Learn more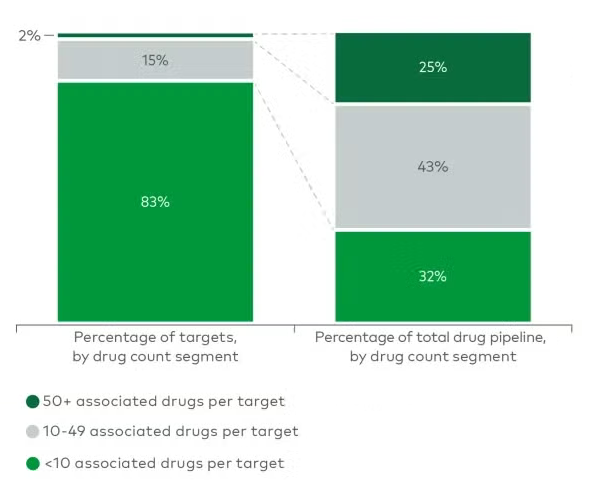
Biopharma Drug Development Gaps Strategy
Why biopharma drug development is stalling around just 38 targets? Novel targets decline as biopharma companies favors the familiar ones. Expert report reveals risk-averse shift in biopharma pipeline. Known targets offer efficiency, predictability, and infrastructure. Target crowding threatens innovation in Biopharma Drug Development.
Learn more
Winning Trust Through Mabion Biologics CDMO Excellence – exclusive interview with Marty Henehan
What does it take to stand out as a global biologics CDMO? In this interview, Marty Henehan shares insights from DCAT Week, discusses Mabion’s award-winning approach to customer partnerships, and reveals how the company is shaping the future of biologics manufacturing.
Learn more