Metabolite and nutrient analysis as crucial components for optimal CHO cell culture upstream process
Cell culture, Drug substance, Manufacturing
- CHO cell culture metabolites can be considered as a class of naturally occurring compounds that act as carriers, substrates or products in biochemical pathways.
- Nutrients essential for cell growth and production of therapeutic antibodies are delivered to the cell culture with medium and feeds. They participate as substrates in metabolic pathways.
- The concentrations of metabolites vary strongly depending on the nutrient input and cellular responses to environmental changes.
- Optimizing the composition of culture media and feeds has a direct and positive impact on bioprocess duration, productivity and purification as well as protein quality.
Background
Mammalian cells, in particular Chinese Hamster Ovary (CHO) cells, are the preferred host for the production of therapeutic proteins such as monoclonal antibodies (mAbs) due to their ability to make posttranslational modifications. Modifications such as glycosylation are essential for biological efficacy and safety [1,2,3] and are considered to be a critical quality attribute for multiple biopharmaceuticals [4]. CHO cells are being used for the production of 84% of FDA-approved biotherapeutics in 2018 [3]. Processes for the production of recombinant proteins in CHO cells have steadily improved through advances in cell line development, clone screening and isolation, and optimization of media and supplements, as well as enhanced process monitoring and control. Optimized feeding compositions and strategies improve culture stability and lead to more efficient cell metabolism [5].
Metabolites can be considered as a class of naturally occurring compounds that act as carriers, substrates or products in biochemical pathways [6]. The field of knowledge that involves the intracellular and extracellular quantification of metabolites is called metabolomics. The concentrations of metabolites strongly vary depending on the cellular responses to environmental changes [3]. Advances in technologies and analytical methods have allowed the routine analysis of the metabolome of mammalian cells. By measuring what is happening in the cells surrounding environment, a detailed picture of the metabolic state can be obtained, which is used as basis for rational optimization of cell culture processes and medium [7]. Medium composition and development of an appropriate feed strategy is essential, as excessive nutrient concentrations can be detrimental to cells due to component concentration dependent toxicity and high osmolality [5].
Components of mammalian cell culture media and supplements
Cell culture media requires essential nutrients that are necessary to sustain cell life and growth [8]. Advances in the development of culture media for the biopharmaceutical production of CHO cells have led to the current use of fully chemically defined growth media containing substances of non-animal origin. The composition of many culture media is not normally disclosed for commercial reasons. In fact, optimizing the composition of culture media and feeds has a direct and positive impact on bioprocess duration, productivity, purification, and protein quality, ultimately resulting in lower process costs. The feeds are designed to replenish depleted nutrients in the basal medium, alleviating various cellular stresses and supporting cell growth and recombinant protein production [9].
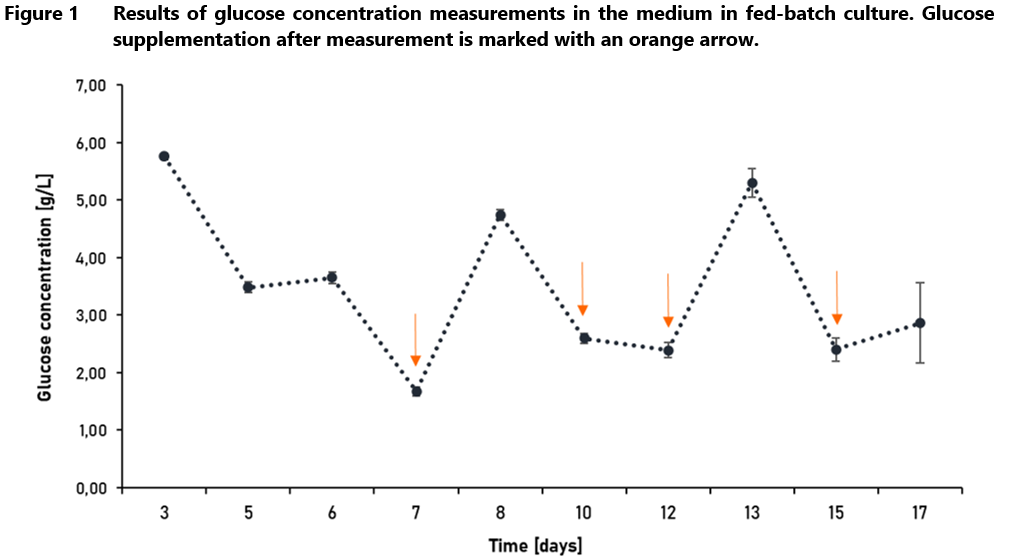
In most media formulations, glucose functions as the primary energy and carbon source [4,8]. Due to the rapid growth and nutrient consumption of CHO cells in the mAb production medium, the glucose level in the medium is usually controlled at higher levels than 3 mM (0.540 g/L), at which it has been shown that CHO cells can maintain high viability [8]. Glucose supplementation based on the concentration measured on planned days of culture is shown in Figure 1. The purpose of this type of supplementation is to maintain the appropriate glucose level for cell growth and viability.
Alternative carbon sources that can be used in CHO cell culture media are galactose, mannose or other hexoses. Replacing glucose with another sugar allows to achieve different effects. For example, replacing glucose with mannose in the medium can increase volumetric productivity by accelerating cell growth and reducing lactate accumulation without changing product quality. In addition to glucose and other hexose sugars, glutamine is also utilized as a major energy source in CHO cell culture and its absence tends to delay the start of the exponential growth phase. Supplementation of glutamine in basal media has been reported to improve cell viability, increase antibody productivity and reduce lactate production [8].
Studies have shown that small changes in the amino acid composition of the cell culture medium can alter the growth and titer profiles and can significantly affect the product’s glycosylation patterns. In addition to increasing the titer and peak density of cells, selected amino acids at certain concentrations may have a protective effect on growing cells [8].
Lipids are major components of biological membranes, Golgi apparatus and endoplasmic reticulum, and can also serve as energy sources and signaling molecules in mammalian cells. Generally, CHO cells are able to synthesize lipids on their own. Although recombinant CHO cells are able to grow in a lipid-free medium [8], supplementation with this ingredient has been shown to be beneficial for cell viability and glycosylation [8,10].
Vitamins are organic compounds that act as coenzymes, prosthetic groups or cofactors in the signaling cascade, as well as in the inhibition and activation of enzymes. Despite the required trace amounts, vitamins are essential components of cell culture media and allow for higher mAb productivity and cell growth [8, 10]. The high reducing capacity of vitamins can protect cells against oxidative radicals [8]. They can also inhibit the acidic variants while enhancing basic variants [10]. Many commercial media contain B vitamins and their derivatives. However, not all vitamins are essential to the process. For example, supplementation with folic acid, cobalamin, biotin and 4-aminobenzoic acid has been shown to be as effective in increasing cell growth and productivity as increasing the concentration of all vitamins in the medium [8].
Other components added to growth media include salts, trace elements and growth factors. Salts maintain the potential of the biological membrane, are responsible for providing appropriate osmolality and buffering. Growth factors are typically peptides, small proteins and hormones that act as signaling molecules affecting cell growth, differentiation and proliferation. Trace elements added in small quantities play roles in the regulation of metabolic pathways [8].
Metabolism of mammalian cells – main biosynthetic and catabolic pathways of CHO cells
Industrial scale production of recombinant therapeutic proteins such as monoclonal antibodies (mAb), is predominantly performed in fed-batch systems. In these systems, small volumes of highly concentrated nutrient feeds are delivered to support growth and cell viability [2]. In this type of cultivation CHO cells transition into distinct metabolic phases: initial growth phase and later stationary phase [2,3]. The initial (exponential) phase is characterized by a steep increase in the viable cell density. In this time, the cells use the nutrients contained in the medium, from which catabolism generates energy and various substrates are used for biomass generation [3]. In the growth phase, the mAb production is low, however in the stationary phase recombinant protein production is elevated and cell growth has slowed or stopped [2,3].
The exponential phase of growth corresponds to a state of high consumption in particular glucose and glutamine [3]. Glucose is taken up by the cell at high rates, phosphorylated to glucose-6-phosphate (G6P) and used in glycolysis to form adenosine triphosphate (ATP), reduced nicotinamide adenine dinucleotide (NADH) and pyruvate. Instead of proceeding to the full oxidation of glucose in aerobic conditions, pyruvate is converted into lactate [4]. At the beginning of cell culture, the concentration of glucose in the medium has no significant effect on the rate of lactate production, while in later stages, high glucose concentrations may result in increased lactate accumulation [1,4,8]. Metabolite analysis showed that in the initial phase, CHO cells largely generate energy through aerobic glycolysis and produce lactate regardless of oxygen concentration, while stationary phase cells mainly perform oxidative phosphorylation and consume lactate. Glutamine is metabolized via glutaminolysis and enters the Krebs cycle in the form of α-ketoglutarate. During glutaminolysis, glutamine is usually converted to glutamate, thereby also producing free ammonium in the medium, which is thought to reduce the rate of cell growth [8].
In CHO cell culture, amino acids are provided in the growth medium and/or produced by biosynthesis. Through the catabolism of amino acids, the cell can use carbon backbones to create intermediates of the Krebs cycle to be used in central metabolic pathways. Providing amino acids in excess leads to the formation of by-products such as ammonium or ammonia.
Impact of metabolites on cell culture and drug product
Studies and observations of CHO cell metabolism have led to many conclusions in the literature. Knowledge about the metabolism of the cells in which the therapeutic protein is produced, allows for better understanding and thus controlling the manufacturing process, taking under consideration critical process parameters, which follow the Process Analytical Technology (PAT) approach of the Food and Drug Administration (FDA) [11]. Metabolomics is also well suited to monitoring the quality of raw materials [7], which is in line with the ICH Q11 guideline stating that “the manufacturing process development program should identify which material attributes (e.g., of raw materials, starting materials, reagents, solvents, process aids, intermediates) and process parameters should be controlled” [13]. Some of the metabolites formed in the metabolic pathways described above have negative effects on cell cultures. That is why it is so important to adjust the composition of the medium and supplements used in the process to the cell’s specific metabolic requirements based on previous studies of the cell line [4]. Examples of such studies are presented in Figure 2 and Figure 3, which show the impact of individual media on cell density and viability.
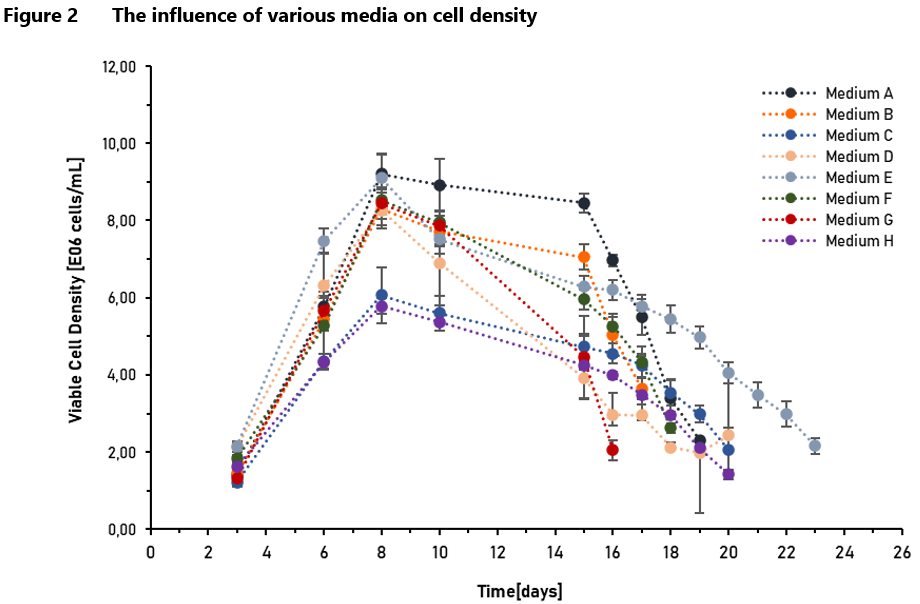
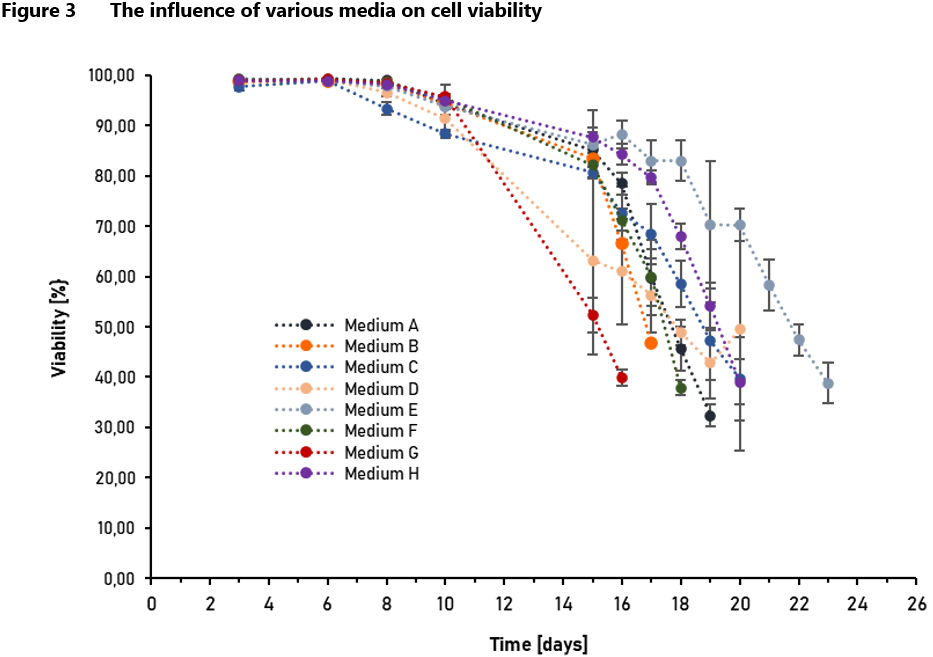
Consumption of glucose usually leads to pyruvate accumulation and an increase in lactate concentration [8]. Reports have shown that lactate inhibits cell growth, induces apoptosis and reduces productivity of recombinant therapeutic products, due to changes in pH and osmolality [4]. These observations have led to the development of strategies utilizing low or limited glucose concentration in the culture media [8]. During cell culture, the minimum glucose concentration should be controlled at 2 ~ 3 g/L [10].
For CHO cells, cell growth inhibition has been reported for ammonia concentration of 5.1 mM and a reduction of 50% of growth was observed if ammonia concentrations exceeded 8 mM [4]. High ammonium concentration in the medium can have a significant effect on cell culture by increasing the glycoform heterogeneity and affecting the consumption rate of other amino acids [8].
Notably, differences in CHO cell metabolism studies can be observed, and explained by the unique features of CHO cell lines derived from a single host, acquired as a result of genetic drift in separate laboratories, and differences in the media and feeding strategies used [3].
Summary
Understanding of the processes that occur in living cells facilitates the development of an optimal and highly efficient cell culture process. Regular monitoring of nutrients and metabolites allows for adequate supplementation of the media and maintaining their concentration at appropriate levels throughout the entire cell culture. Measurements also allow for checking the media and supplements before starting the process.
Literature
1. Buchsteiner, M., Quek, L.-E., Gray, P., & Nielsen, L. K. (2018). Improving culture performance and antibody production in CHO cell culture processes by reducing the Warburg effect. Biotechnology and Bioengineering. doi:10.1002/bit.26724
2. Dean J, Reddy P. Metabolic analysis of antibody producing CHO cells in fed-batch production. Biotechnol Bioeng. 2013 Jun;110(6):1735-47. doi: 10.1002/bit.24826. Epub 2013 Feb 15. PMID: 23296898.
3. Coulet M, Kepp O, Kroemer G, Basmaciogullari S. Metabolic Profiling of CHO Cells during the Production of Biotherapeutics. Cells. 2022 Jun 15;11(12):1929. doi: 10.3390/cells11121929. PMID: 35741058; PMCID: PMC9221972.
4. Pereira S, Kildegaard HF, Andersen MR. Impact of CHO Metabolism on Cell Growth and Protein Production: An Overview of Toxic and Inhibiting Metabolites and Nutrients. Biotechnol J. 2018 Mar;13(3): e1700499. doi: 10.1002/biot.201700499. Epub 2018 Feb 19. PMID: 29393587.
5. Reinhart, D., Damjanovic, L., Kaisermayer, C. et al. Benchmarking of commercially available CHO cell culture media for antibody production. Appl Microbiol Biotechnol 99, 4645–4657 (2015). Doi:10.1007/s00253-015-6514-4
6. Khoo SH, Al-Rubeai M. Metabolomics as a complementary tool in cell culture. Biotechnol Appl Biochem. 2007 Jun;47(Pt 2):71-84. doi: 10.1042/BA20060221. PMID: 17492944.
7. Dietmair S, Hodson MP, Quek LE, Timmins NE, Chrysanthopoulos P, Jacob SS, Gray P, Nielsen LK. Metabolite profiling of CHO cells with different growth characteristics. Biotechnol Bioeng. 2012 Jun;109(6):1404-14. doi: 10.1002/bit.24496. Epub 2012 Mar 30. PMID: 22407794.
8. Cell Culture Media for Recombinant Protein Expression in Chinese Hamster, Ovary (CHO) Cells: History, Key Components, and Optimization Strategies Frank V. Ritacco, Yongqi Wu, Anurag Khetan, DOI 10.1002/btpr.2706
9. Pérez-Rodriguez S, Ramírez-Lira MJ, Trujillo-Roldán MA, Valdez-Cruz NA. Nutrient supplementation strategy improves cell concentration and longevity, monoclonal antibody production and lactate metabolism of Chinese hamster ovary cells. Bioengineered. 2020 Dec;11(1):463-471. doi: 10.1080/21655979.2020.1744266
10. Xu WJ, Lin Y, Mi CL, Pang JY, Wang TY. Progress in fed-batch culture for recombinant protein production in CHO cells. Appl Microbiol Biotechnol. 2023 Feb;107(4):1063-1075. doi: 10.1007/s00253-022-12342-x. Epub 2023 Jan 17. PMID: 36648523; PMCID: PMC9843118.
11. André S, Lagresle S, Da Sliva A, Heimendinger P, Hannas Z, Calvosa É, Duponchel L. Developing global regression models for metabolite concentration prediction regardless of cell line. Biotechnol Bioeng. 2017 Nov;114(11):2550-2559. doi: 10.1002/bit.26368. Epub 2017 Aug 29. PMID: 28667738.
13. Q11 Step 5 Development and manufacture of drug substances (europa.eu)
Prepared by:
Małgorzata Nosek
Olga Grzyb
Adam Tuszyner

If you are interested in our upstream development and culture media testing services, please do not hesitate to contact us. Our scientists are ready to support you in end-to-end design of the upstream manufacturing process, from clone selection to optimization of product expression. We also offer fully automated, high-throughput analysis of media constituents and metabolites secreted by cultured cells. Click here to obtain more information.
Related resources
Mass spectrometry in peptide and protein analysis
Analytics, Drug substance, Proteins
Time to Market Strategy – 5 CDMO Tactics to Speed Up Biologic Launches
Biologics, Drug development, Manufacturing

Navigating OOX Results: Effective Analysis and Management in CDMO Laboratories
Analytics, Manufacturing, Regulatory